- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Acute Cerebellar Slice Preparation Using a Tissue Chopper
Published: Vol 9, Iss 5, Mar 5, 2019 DOI: 10.21769/BioProtoc.3187 Views: 5776
Reviewed by: Jackeline Moraes MalheirosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3756 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2625 Views

Ultrafast Isolation of Synaptic Terminals From Rat Brain for Cryo-Electron Tomography Analysis
Rong Sun and Qiangjun Zhou
Sep 5, 2025 3555 Views
Abstract
Acute cerebellar slices are widely used among neuroscientists to study the properties of excitatory and inhibitory synaptic transmission as well as intracellular signaling pathways involved in their regulation in cerebellum. The cerebellar cortex presents a well-organized circuitry, and several neuronal pathways can be stimulated and recorded reliably in acute cerebellar slices. A widely used acute cerebellar slice preparation technique was adapted from Edwards’ thin slice preparation method published in 1989 (Edwards et al., 1989). Most of the acute cerebellar slice preparation techniques use a vibrating microtome for slicing freshly dissected cerebellum from various animal species. Here we introduce a simpler method, which uses a tissue chopper to quickly prepare acute sagittal cerebellar slices from rodents. Cerebellum is dissected from the whole brain and sliced with a tissue chopper into 200-400 µm thick slices. Slices are allowed to recover in oxygenated aCSF at 37 °C for 1-2 h. Slices can then be used for electrophysiology or other types of experimentation. This method can be used to prepare cerebellar slices from mouse or rat aged from postnatal day 7 to 2 years. The preparation is faster and easier than other methods and provides a more versatile diversity of applications.
Keywords: Acute cerebellar sliceBackground
Acute cerebellar slice preparations, like many other current brain slice preparation techniques, originated from Edwards’ thin slice preparation method published in 1989 (Edwards et al., 1989). In general, cerebellum was quickly dissected and immersed in Ca2+-free aCSF and glued to the stage of a vibratome. After slicing, slices were recovered in regular aCSF for 1-2 h before use (Llano et al., 1991a and 1991b; Kano and Konnerth, 1992). Here we introduce a method adapted from the acute hippocampal slice preparation method used in our laboratory. Freshly dissected cerebellum is rapidly sliced (around 10 s) on a McIlwain tissue chopper without the need for glue or oxygenation during slicing. The slices prepared using this method are as healthy as the ones prepared with a vibratome and can be used for electrophysiology or a variety of other manipulations.
Materials and Reagents
- Disposable pipette (VWR, catalog number: 16001-180)
- Parafilm (VWR, catalog number: 52858-000)
- 60 mm and 100 mm Petri dishes (VWR, catalog numbers: 25373-085 and 25373-100)
- Filter paper (VWR, catalog number: 28460-030)
- Painting brushes
- Gas dispersion tube (Ace Glass, catalog number: 9435-10)
- Mice
- Adult C57BL/6 (WT) mice
- Adult calpain-1 KO mice
- Adult calpain-1 PHLPP1 double-KO (DKO) mice
- Isofluran
- NaCl
- KCl
- CaCl2
- MgSO4
- D-glucose
- NaHCO3
- KH2PO4
- Artificial cerebrospinal fluid (aCSF) (see Recipes)
- Cutting solution (see Recipes)
Equipment
- Chill dissection tools
- Angled-tip dissector scissor (Fine Science Tools, catalog number: 14082-09)
- Scissor (Fine Science Tools, catalog number: 91401-14)
- Curved-tip forceps (Fine Science Tools, catalog number: 11051-10)
- Surgical blade
- Spoon
- Dissecting spatula
- Brain slice keeper (AutoMate Scientific, catalog number: BSK4)
- Beakers
- Ice box
- McIlwain Tissue Chopper (The Mickle Laboratory Engineering, Brinkmann)
- Water bath
- 95% O2/5% CO2 air tank
- -80 °C freezer
Procedure
- Prepare 1 L of artificial cerebrospinal fluid (aCSF) (see Recipe 1).
- Pour 500 ml of freshly prepared aCSF into a brain slice container. Put the brain slice container in a 37 °C water bath. Connect the brain slice container to a 95% O2/5% CO2 air tank and oxygenate aCSF. Gas flow pressure is set at around 2 p.s.i.
- Pour 100 ml of cutting solution into a 150 ml beaker. Cover the beaker with parafilm, and place it in a -80 °C freezer for 5-10 min, until the solution has a slushy consistency.
- Place the ice-cold cutting solution on a large ice tray. Bubble the solution with a gas dispersion tube connected to a 95% O2/5% CO2 air tank for 5 min.
- Place the lid of a 60 mm Petri dish on ice. Put a round filter paper onto the lid and wet the filter paper with the cutting solution. This is the brain dissection stage.
- Chill dissection tools including angled-tip dissector scissors, large scissors, a curved-tip forceps, a surgical blade, a spoon, a disposable pipette with the tip cut off and two dissecting spatulas on ice. The whole dissection setup is shown in Figure 1.
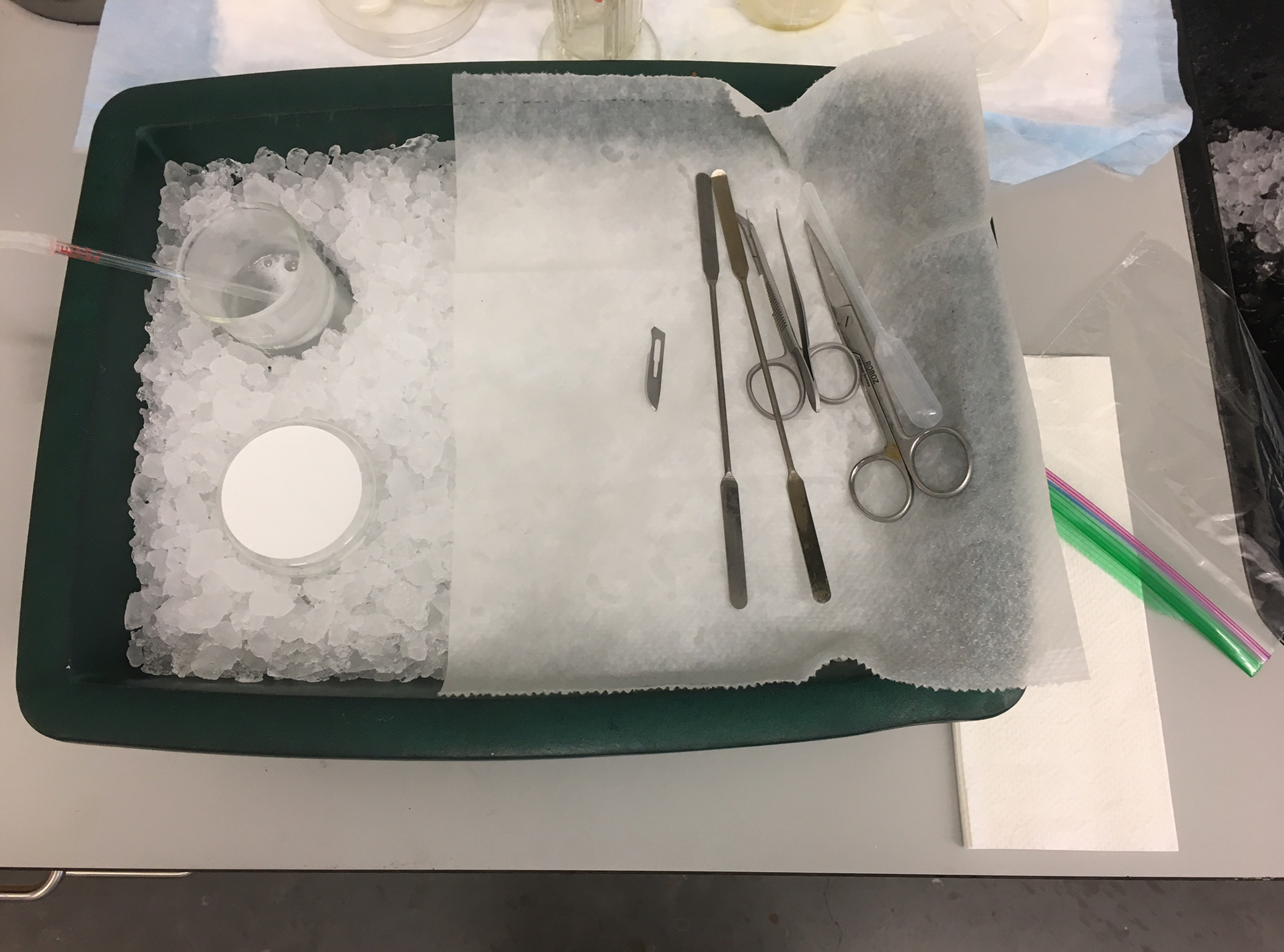
Figure 1. Dissecting setup for acute cerebellar slice preparation. A beaker with cutting solution, a dissection stage, and dissection tools were placed on a large ice tray. - Anesthetize animal with isoflurane and decapitate with the appropriate tool (e.g., scissors). Immediately extract the whole brain with dissection tools and place it into the ice-cold cutting solution. This step should be done within 1 min.
- Chill the brain in ice-cold cutting solution for 1 min.
- Place the brain onto the cold dissection stage. First, cut off forebrain, midbrain, and medulla. Then, cut off pons to isolate the cerebellum (Figure 2).
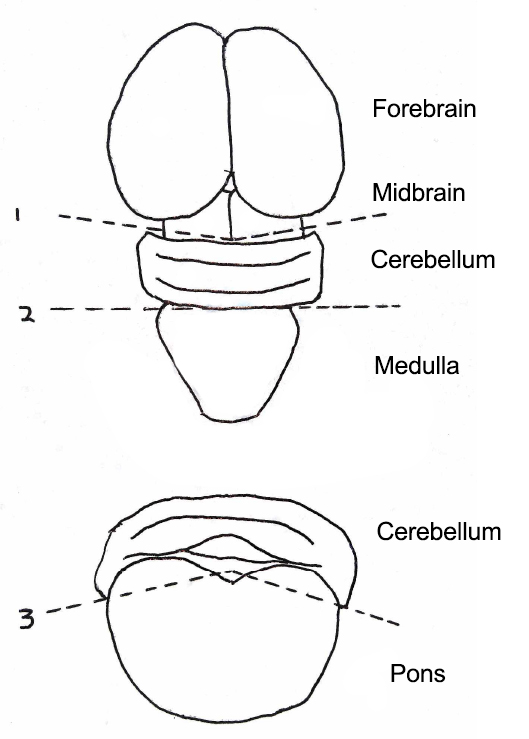
Figure 2. Steps to isolate cerebellum from whole mouse brain. Forebrain, midbrain, and medulla were cut off, followed by the removal of pons beneath the cerebellum. Top, the axial view of mouse brain. Bottom, the coronal view of Cerebellum and Pons. Numbers and dotted lines indicate steps and sites of the incision. - Immediately transfer the cerebellum onto the stage of a McIlwain Tissue Chopper. Drain the extra solution around the tissue. Slice the cerebellum into 200-400 µm thick sagittal slices (Figure 3).

Figure 3. McIlwain Tissue Chopper (The Mickle Laboratory Engineering, Brinkmann) - Move the cerebellum to a 100 mm Petri dish containing ice-cold cutting solution and carefully separate each slice without damaging them using two small painting brushes.
- Transfer slices into warm aCSF in the brain slice container using a disposable pipette with the tip cut off.
- Incubate slices in aCSF at 37 °C for 1-2 h with the 95% O2/5% CO2 bubbling on. Slices can now be used for electrophysiology or slice treatment.
Data analysis
We prepared acute cerebellar slices of 3 months old mice following the protocol we described here and recorded excitatory postsynaptic potentials (EPSPs) elicited in the Purkinje cell body layer by electrical stimulation of the parallel fibers at various stimulation intensities (Wang et al., 2016). This stimulation elicited a typical P1-N1-P2-N2 waveform (Barnes et al., 2011), where N1 corresponds to the presynaptic fiber volley and N2 to the postsynaptic population spike (Figure 4). Responses elicited by stimulation intensity below 120 mA were too unreliable to be analyzed, and only responses elicited by stimulation intensities above 120 mA were analyzed by calculating the ratio of N2 over N1, which reflects the efficiency of synaptic transmission. At all intensities, the N2/N1 ratio was smaller in calpain-1 KO mice as compared with WT mice, and the overall difference between the two genotypes was statistically significant (Figure 4). The N2/N1 ratios of the EPSPs recorded in cerebellar slices from calpain-1, and PHLPP1 double-KO (DKO) mice at all stimulation intensities were intermediate between those in WT and calpain-1 KO mice, indicating that synaptic transmission was at least partially restored in the cerebellum of DKO mice (Figure 4).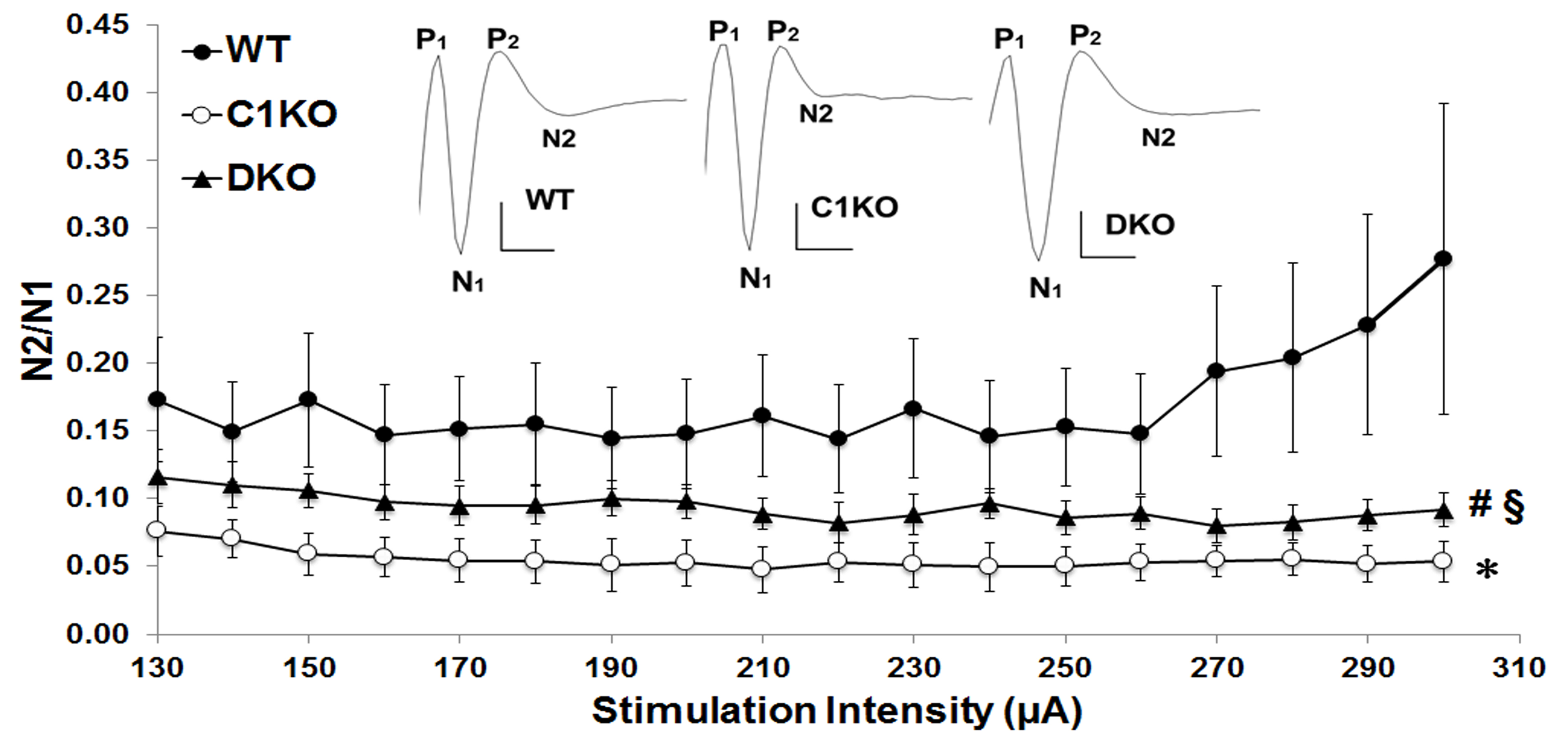
Figure 4. Parallel fibers to Purkinje cell EPSPs are reduced in adult calpain-1 KO mice, and partially restored in adult calpain-1 and PHLPP1 double-KO (DKO) mice. Acute cerebellar slices were prepared as described in Procedure, and field EPSPs were evoked by parallel fiber stimulation recorded in the Purkinje cell layer. Results were calculated as ratios of N2 over N1 and represent means ± SEM of 10 to 11 slices from three to five mice. *P < 0.001, as compared with WT (univariate ANOVA followed by Bonferroni test); #P < 0.001, as compared with WT (univariate ANOVA followed by Bonferroni test); §P < 0.001, as compared with DKO (univariate ANOVA followed by Bonferroni test) (from Wang et al., 2016).
Recipes
- Artificial cerebrospinal fluid (aCSF)
110 mM NaCl
5 mM KCl
2.5 mM CaCl2
1.5 mM MgSO4
1.24 mM KH2PO4
10 mM D-glucose
27.4 mM NaHCO3 - Cutting solution
124 mM NaCl
26 mM NaHCO3
10 mM glucose
3 mM KCl
1.25 mM KH2PO4
5 mM MgSO4
3.4 mM CaCl2
Cutting solution can be stored at 4 °C for 1 week
Acknowledgments
This work was supported by grant P01NS045260-01 from NINDS (PI: Dr. C.M. Gall) and grant R01NS057128 from NINDS to MB.
Competing interests
There are no conflicts of interest.
Ethics
Animal use in all experiments followed NIH guidelines and all protocols were approved by theInstitution Animal Care and Use Committee of Western University of Health Sciences.
References
- Barnes, J. A., Ebner, B. A., Duvick, L. A., Gao, W., Chen, G., Orr, H. T. and Ebner, T. J. (2011). Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1[82Q] mice. J Neurosci 31(36): 12778-12789.
- Edwards, F. A., Konnerth, A., Sakmann, B. and Takahashi, T. (1989). A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch 414(5): 600-612.
- Kano, M., and Konnerth, A. (1992). Cerebellar slices for patch clamp recording. In: Kettenmann, H. and Grantyn, R. (Eds.). Practical electrophysiological methods. pp: 54-57.
- Llano, I., Dreessen, J., Kano, M. and Konnerth, A. (1991a). Intradendritic release of calcium induced by glutamate in cerebellar Purkinje cells. Neuron 7(4): 577-583.
- Llano, I., Marty, A., Armstrong, C. M. and Konnerth, A. (1991b). Synaptic- and agonist-induced excitatory currents of Purkinje cells in rat cerebellar slices. J Physiol 434: 183-213.
- Wang, Y., Hersheson, J., Lopez, D., Hammer, M., Liu, Y., Lee, K. H., Pinto, V., Seinfeld, J., Wiethoff, S., Sun, J., Amouri, R., Hentati, F., Baudry, N., Tran, J., Singleton, A. B., Coutelier, M., Brice, A., Stevanin, G., Durr, A., Bi, X., Houlden, H. and Baudry, M. (2016). Defects in the CAPN1 gene result in alterations in cerebellar development and cerebellar ataxia in mice and humans. Cell Rep 16(1): 79-91.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, Y. and Baudry, M. (2019). Acute Cerebellar Slice Preparation Using a Tissue Chopper. Bio-protocol 9(5): e3187. DOI: 10.21769/BioProtoc.3187.
Category
Neuroscience > Basic technology > Acute slice preparation
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link