- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantification of Serum Ovalbumin-specific Immunoglobulin E Titre via in vivo Passive Cutaneous Anaphylaxis Assay
Published: Vol 9, Iss 5, Mar 5, 2019 DOI: 10.21769/BioProtoc.3184 Views: 6242
Reviewed by: Lokesh KalekarVishal NehruPorkodi Panneerselvam

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A High-throughput Automated ELISA Assay for Detection of IgG Antibodies to the SARS-CoV-2 Spike Protein
Juliana Conkright-Fincham [...] Jay R. Unruh
Jan 20, 2022 4603 Views

Human Auto-IgG Purification from High Volume Serum Sample by Protein G Affinity Purification
Serena Sensi and Andreas Goebel
Dec 5, 2022 2832 Views

Determination of Antibody Activity by Platelet Aggregation
Halina H.L. Leung [...] Beng H. Chong
Sep 5, 2023 2255 Views
Abstract
Murine models of allergic airway disease are frequently used as a tool to elucidate the cellular and molecular mechanisms of tissue-specific asthmatic disease pathogenesis. Paramount to the success of these models is the induction of experimental antigen sensitization, as indicated by the presence of antigen-specific serum immunoglobulin E. The quantification of antigen-specific serum IgE is routinely performed via enzyme-linked immunosorbent assay. However, the reproducibility of these in vitro assays can vary dramatically in our experience. Furthermore, quantifying IgE via in vitro methodologies does not enable the functional relevance of circulating IgE levels to be considered. As a biologically appropriate alternative method, we describe herein a highly reproducible in vivo passive cutaneous anaphylaxis assay using Sprague Dawley rats for the quantification of ovalbumin-specific IgE in serum samples from ovalbumin-sensitized murine models. Briefly, this in vivo assay involves subcutaneous injections of serum samples on the back of a Sprague Dawley rat, followed 24 h later by intravenous injection of ovalbumin and a blue detection dye. The subsequent result of antigen-IgE mediated inflammation and leakage of blue dye into the initial injection site indicates the presence of ovalbumin-specific IgE within the corresponding serum sample.
Keywords: AsthmaBackground
Allergic asthma is a complex inflammatory disease, the development of which involves multiple intricate gene-environment interactions. A defining factor of the genetic component for disease inception is the predisposition to developing allergen-specific immunoglobulin (Ig) E to common allergens, otherwise known as atopy (Sly et al., 2008). As such, allergic (atopic) sensitization to innocuous perennial aeroallergens during early childhood is now recognized as a fundamental risk factor in the establishment of potentially life-long allergic asthmatic disease (Illi et al., 2006; Sly et al., 2008), particularly when sensitization occurs to multiple aeroallergens (Stoltz et al., 2013).
Murine models have long provided a means to investigate the cellular and molecular mechanisms that drive onset and progression of allergic airway diseases such as allergic asthma (Kumar et al., 2016). In this regard, mice are typically experimentally sensitized to a model antigen, resulting in CD4+ T-helper (Th) type 2-driven inflammation and the production of antigen-specific IgE (Mincham et al., 2018); the hallmark feature of allergic sensitization. Quantification of circulating serum antigen-specific IgE from antigen sensitized murine models is routinely performed by enzyme-linked immunosorbent assay (ELISA). However, in our experience, these in vitro assays lack the necessary reproducibility for accurate detection of ovalbumin (OVA)-specific serum IgE in both Brown Norway rat and BALB/c mouse models of allergic airway disease. Furthermore, the use of in vitro techniques disregards the functional activity of antigen-specific IgE in a biologically relevant setting.
To combat the disadvantages of in vitro IgE quantification assays, we herein detail a highly reproducible in vivo passive cutaneous anaphylaxis (PCA) assay which enables both the quantification and assessment of biological activity of OVA-specific IgE within serum samples from OVA-sensitized murine models. In this regard, the localized cutaneous anaphylaxis response observed from an OVA-specific IgE positive sample is indicative of mast cell degranulation driven via OVA-induced cross-linking of OVA-specific IgE (Kawakami and Galli, 2002). The resultant release of mast cell granule-derived proinflammatory mediators exemplified by histamine, prostaglandin, leukotriene and tryptase, ultimately stimulates vascular permeability and the subsequent extravasation of the blue detection dye (Bradding and Arthur, 2016). While this protocol has been described for quantifying OVA-specific IgE in murine serum samples, it could be adapted to assess alternative model antigens.
Materials and Reagents
- 1.5 ml screw cap micro tube (SARSTEDT, catalog number: 72.703.600)
- 5 ml screw cap tube (SARSTEDT, catalog number: 60.9921.524)
- 27 G x ½” 1 ml Insulin syringe (Terumo, catalog number: SS-10M2713A)
- 26 G x ½” needle (Terumo, catalog number: NN-2613R)
- 23 G x 1¼” needle (Terumo, catalog number: NN-2332R)
- 3 ml syringe (Terumo, catalog number: SS-03L)
- 5 ml syringe (Terumo, catalog number: SS-05L)
- 80 μm syringe filter (Sartorius, Minisart®, catalog number: 16592)
- 45 μm syringe filter (Sartorius, Minisart®, catalog number: 16555)
- 96-well microplate (Thermo Fisher Scientific, NuncTM MicroWellTM, catalog number: 260860)
- Cotton wool roll (BNS Medical, catalog number: 71841-14)
- Marker pen
- Aluminum foil
- Male Sprague Dawley rats > 10 weeks old (Animal Resource Centre, Murdoch, Australia)
- Serum samples derived from ovalbumin-sensitized murine models
- Evans blue (Sigma-Aldrich, catalog number: E2129-10G), store at room temperature
- Ovalbumin (OVA) lyophilized powder (Sigma-Aldrich, catalog number: A5503), store at 4 °C
- Sterile Water for Irrigation (Baxter, catalog number: 2F7114), store at room temperature
- Sterile Water for Injection (Pfizer, catalog number: 25020010), store below 25 °C
- Chloral hydrate crystalized (Sigma-Aldrich, catalog number: 23100-250G)
- Sterile Phosphate buffered saline (PBS) (made in house)
- Topical ophthalmic ointment (Novartis, Viscotears®, catalog number: 1AD031591)
- Pentobarbitone sodium (Virbac, Lethabarb®, catalog number: LETH-1)
- Isoflurane (Henry Schein, IsoThesia®, catalog number: 988-3244), store below 25 °C in the dark
- 5.71% chloral hydrate solution (C2H3Cl3O2) (see Recipes)
- 1% Evans Blue dye stock solution (see Recipes)
- 10 mg/ml ovalbumin stock solution (see Recipes)
- 1:1 Ovalbumin-Evans blue dye solution for one rat (see Recipes)
Equipment
- 100 ml Schott bottle (Sigma-Aldrich, Duran®, catalog number: Z305170)
- 500 ml Bottle Top Filter, 0.2 μm pore size (Thermo Fisher Scientific, Nalgene® Rapid-FlowTM, catalog number: 595-4520)
- Animal hair clippers (Wahl, KM CordlessTM, catalog number: WA-9596-212)
- Warming pad (Kent Scientific Corporation, catalog number: DCT-25)
- Small animal anesthesia system (Kent Scientific Corporation, SumnoSuite®, catalog number: SS-01)
- 4 °C refrigerator
- -20 °C freezer
Software
- GraphPad Prism (GraphPad software, version 7.0a)
Procedure
- Serial 1 in 2 dilutions of serum samples
- Using a 96-well microplate, perform serial 1 in 2 dilutions of serum in PBS to a final sample volume of 55 μl.
Notes:- Serum samples are obtained from OVA-sensitized and aerosol challenged murine models.
- Final serum dilutions include: neat, 1:2, 1:4, 1:8, 1:16, 1:32, 1:64 and 1:128 samples.
- Duplicate 55 μl PBS only samples are required as negative controls.
- A total of 8 individual experimental samples can be analyzed per rat, in addition to duplicate negative control samples.
- Using a 96-well microplate, perform serial 1 in 2 dilutions of serum in PBS to a final sample volume of 55 μl.
- Male Sprague Dawley rat anesthetization
- Place male Sprague Dawley rat in an anesthetic induction chamber and anesthetize via isoflurane for approximately 3 min.
- Once sufficiently anesthetized, intraperitoneally inject the male Sprague Dawley rat with 4 ml 5.71% chloral hydrate solution using a 23 G x 1¼” needle and 5 ml syringe.
Notes:- This volume of 5.71% chloral hydrate solution will anesthetize an adult male Sprague Dawley rat (> 10 weeks old) for approximately 1 h.
- The male Sprague Dawley rat has reached sufficient deep plane anesthesia when the pedal withdrawal reflex is absent or barely noticeable.
- Place a small amount of topical ophthalmic ointment on the eyes of the anesthetized rat to stop them drying out during the procedure.
- Subcutaneous injections of serum dilutions
- Place the anesthetized male Sprague Dawley rat on a warming pad and closely shave the entire back of the rat from the base of the neck to the hips.
- Using a marker pen, mark an 8 x 8 dot grid covering the entire back of the rat. Space the dots approximately 1 cm apart (Figure 1).
Notes:- Do not use a blue marker as this will be hard to distinguish from the Evans blue dye.
- An adult male Sprague Dawley rat will fit approximately 8 individual serum samples.
- Include 2 additional dots below the 8 x 8 sample grid for duplicate negative control samples.
- Starting with the highest dilution (1:128), subcutaneously inject 50 μl of the sample under the first marked dot using a 27 G x ½” 1 ml Insulin syringe. Continue down the first row of dots injecting each of the serial dilutions.
Note: Start injections from the right-hand side of the rat if you inject with your right hand. This will avoid disturbing the injection sites as you progress across the back (Figure 1). - Subcutaneously inject the 7 remaining individual samples and the duplicate PBS negative control samples, as per Step C3.
Note: Always use a new sterile syringe for each individual sample. - Once all samples have been injected, cover the back of the rat in cotton wool roll and place back in the housing cage.
- Monitor the anesthetized male Sprague Dawley rat periodically until full consciousness is regained.
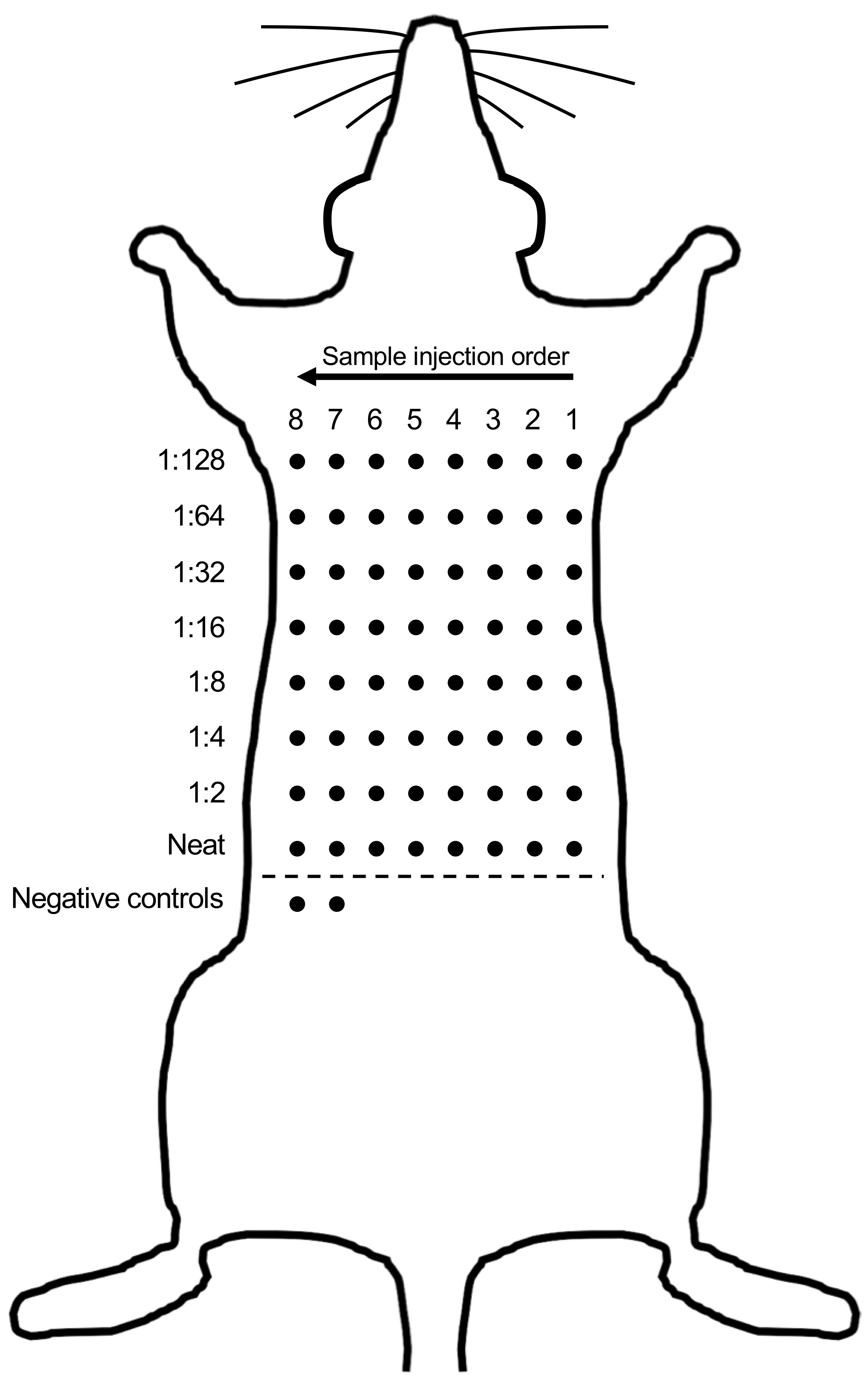
Figure 1. Representative diagram of steps performed in Procedure C
- Evans blue dye intravenous injection
- Twenty-four hours after subcutaneously injecting the serum samples, re-anesthetize the rat as per Procedure B.
- Place anesthetized rat on its back and intravenously inject 2 ml of the 1:1 OVA-Evans blue dye solution (see Recipes) into the penile vein using a 26 G x ½” needle and 3 ml syringe.
- Place rat on its stomach and allow samples to incubate for 15-30 min.
- Following injection, subcutaneous serum samples that are positive for OVA-specific IgE will develop a blue color (Figure 2). Record the highest dilution for each individual serum sample that returns a positive indication.
- Once all data has been recorded, humanely euthanize the male Sprague Dawley rat by intravenously injecting 600 μl of Lethabarb® Pentobarbitone sodium into the heart using a 27 G x ½” 1 ml syringe.

Figure 2. Extravasation of Evans blue detection dye indicating the presence of OVA-specific IgE in the serum sample. Subcutaneously injected serial dilutions of serum samples that contain OVA-specific IgE will become blue following the intravenous injection of an OVA-Evans blue dye solution. Record the highest dilution at which a blue injection site is present for each sample (i.e., 1:4 dilution for Sample 2).
Data analysis
Plot data on a log2 scale to account for the serial dilutions (i.e., neat = 1, 1:2 = 2, 1:4 = 3, 1:8 = 4, 1:16 = 5, 1:32 = 6, 1:64 = 7, 1:128 = 8) using a graphing program such as GraphPad prism. Depending on the number of experimental groups, statistical significance can be determined by Student’s t-test or one-way ANOVA. As detailed in the original research article studying BALB/c mice (Mincham et al., 2018; Figure 1A), by additional research conducted within our laboratory studying Brown Norway rats (Leffler et al., 2018) and in Figure 3 below comparing gender-specific responses following early life OVA sensitization and aerosol challenge (Protocol ID: 1802387), OVA-specific serum IgE titres are significantly greater in OVA sensitized and aerosol challenged animals compared to naïve control animals.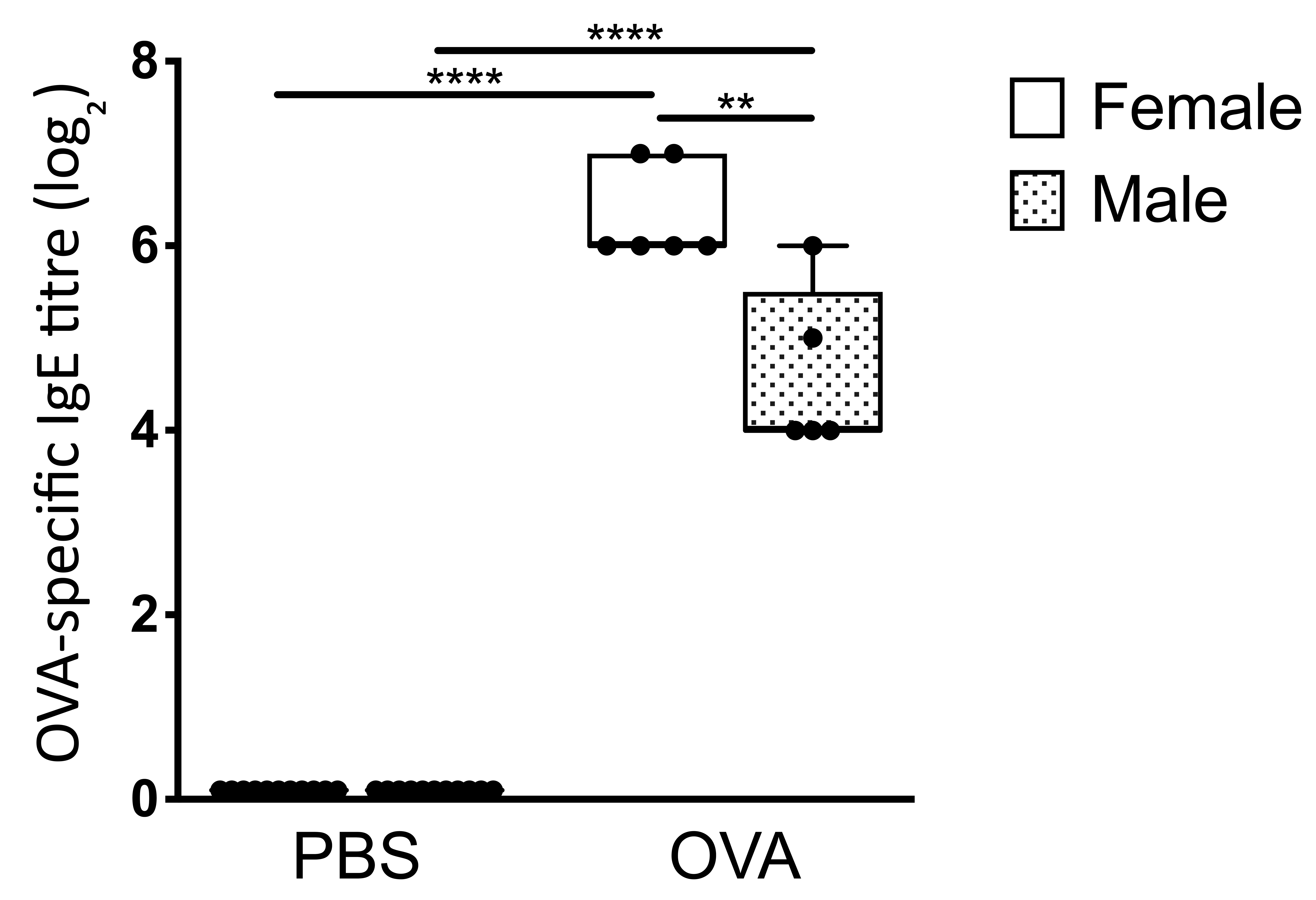
Figure 3. Enhanced serum OVA-specific IgE titers in early life OVA sensitized and aerosol challenge BALB/c mice. Comparison of male and female serum OVA-specific IgE titers as measured by passive cutaneous anaphylaxis assay. Data are presented from individual animals comparing PBS control versus OVA sensitized and aerosol challenged male (shaded) and female (white) BALB/c mice and displayed as box and whisker plots showing minimum to maximum of n ≥ 6 independent experiments. Statistical significance was determined using Student’s t-test and presented as **P < 0.01, ****P < 0.0001.
Recipes
- 5.71% chloral hydrate solution (C2H3Cl3O2)
- Dissolve 5.71 g chloral hydrate in 100 ml sterile water for injection at room temperature
- Cover (100 ml Schott bottle) with aluminum foil to protect from light and store at 4 °C
- 1% Evans Blue dye stock solution
- Dissolve 1 g Evans blue powder in 100 ml PBS
- Store at 4 °C
- 10 mg/ml ovalbumin stock solution
- Dissolve 1 g OVA lyophilized powder in 100 ml sterile water for irrigation by placing lyophilized OVA powder on the water surface and allow to slowly dissolve at room temperature without stirring the solution
- Once dissolved, filter the 10 mg/ml ovalbumin solution into a sterile 100 ml Schott bottle using a 500 ml bottle top filter
- Aliquot the sterile 10 mg/ml OVA stock solution into 1 ml aliquots (1.5 ml screw cap micro tubes)
- Store at -20 °C
- 1:1 Ovalbumin-Evans blue dye solution for one rat
- Add 400 μl of 10 mg/ml OVA stock solution to 600 μl PBS to yield a final concentration of 4 mg/ml OVA solution in a total volume of 1 ml
- Add 1 ml 1% Evans blue dye to 1 ml 4 mg/ml OVA solution
Note: Filter the necessary volume of 1% Evans blue dye stock solution required for the experiment through 80 μm and 45 μm syringe filters prior to use to remove precipitation. - Store at 4 °C until required
Note: Make up the 1:1 OVA-Evans blue dye solution immediately before anesthetizing the male Sprague Dawley rat.
Acknowledgments
The authors would like to acknowledge the animal technicians at the Telethon Kids Institute Bioresources facility. The original research article (Mincham et al., 2018) utilizing this protocol was funded by the National Health and Medical Research Council of Australia.
Competing interests
The authors declare no financial or non-financial competing interests related to this work.
Ethics
All animal experiments relating to this protocol were formally approved by the Telethon Kids Institute Animal Ethics Committee, which operates under guidelines developed by the National Health and Medical Research Council of Australia for the care and use of animals in scientific research.
References
- Bradding, P. and Arthur, G. (2016). Mast cells in asthma--state of the art. Clin Exp Allergy 46(2): 194-263.
- Illi, S., von Mutius, E., Lau, S., Niggemann, B., Gruber, C., Wahn, U. and Multicentre Allergy Study, g. (2006). Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 368(9537): 763-770.
- Kawakami, T. and Galli, S. J. (2002). Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol 2(10): 773-786.
- Kumar, R. K., Herbert, C. and Foster, P. S. (2016). Mouse models of acute exacerbations of allergic asthma. Respirology 21(5): 842-849.
- Leffler, J., Mincham, K. T., Mok, D., Blank, F., Holt, P. G., Stumbles, P. A. and Strickland, D. H. (2018). Functional differences in airway dendritic cells determine susceptibility to IgE-sensitization. Immunol Cell Biol 96(3): 316-329.
- Mincham, K. T., Scott, N. M., Lauzon-Joset, J. F., Leffler, J., Larcombe, A. N., Stumbles, P. A., Robertson, S. A., Pasquali, C., Holt, P. G. and Strickland, D. H. (2018). Transplacental immune modulation with a bacterial-derived agent protects against allergic airway inflammation. J Clin Invest 128(11): 4856-4869.
- Sly, P. D., Boner, A. L., Bjorksten, B., Bush, A., Custovic, A., Eigenmann, P. A., Gern, J. E., Gerritsen, J., Hamelmann, E., Helms, P. J., Lemanske, R. F., Martinez, F., Pedersen, S., Renz, H., Sampson, H., von Mutius, E., Wahn, U. and Holt, P. G. (2008). Early identification of atopy in the prediction of persistent asthma in children. Lancet 372(9643): 1100-1106.
- Stoltz, D. J., Jackson, D. J., Evans, M. D., Gangnon, R. E., Tisler, C. J., Gern, J. E. and Lemanske, R. F., Jr. (2013). Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin Exp Allergy 43(2): 233-241.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Mincham, K. T., Leffler, J., Scott, N. M., Lauzon-Joset, J., Stumbles, P. A., Holt, P. G. and Strickland, D. H. (2019). Quantification of Serum Ovalbumin-specific Immunoglobulin E Titre via in vivo Passive Cutaneous Anaphylaxis Assay. Bio-protocol 9(5): e3184. DOI: 10.21769/BioProtoc.3184.
Category
Immunology > Antibody analysis > Antibody detection
Cell Biology > Cell-based analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









