- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantification of Serotonin in Rice and Insect Pest and its Functional Analysis in Insects Using Artificial Diet Feeding
Published: Vol 9, Iss 4, Feb 20, 2019 DOI: 10.21769/BioProtoc.3173 Views: 5616
Reviewed by: Rainer MelzerHsuan ChouAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Insect Feeding Assays with Spodoptera exigua on Arabidopsis thaliana
Yanrong You [...] Chuanyou Li
Mar 5, 2020 5313 Views

Quantification of Methylglyoxal Levels in Cowpea Leaves in Response to Cowpea Aphid Infestation
Jacob R. MacWilliams [...] Isgouhi Kaloshian
Oct 20, 2020 3499 Views

Image-Based Lignin Detection in Nematode-Induced Feeding Sites in Arabidopsis Roots
Muhammad Amjad Ali and Krzysztof Wieczorek
May 5, 2025 1805 Views
Abstract
Rice is one of the world’s most important crops, but its production suffers from insect pests. Rice brown planthopper (BPH; Nilaparvata lugens Stål) and striped stem borer (SSB, Chilo suppressalis Walker) are the two most serious pests in rice production. We reported that serotonin is an essential mediator in the interaction between rice and insect. Here, we established a method for extraction and determination of serotonin in rice and BPH.
Keywords: SerotoninBackground
Serotonin (5-hydroxytryptamine) is an important signaling molecule in animals. It has also been shown to be distributed widely in plants. In our previous research, we reported that deficiency of serotonin in rice plants enhances its BPH and SSB resistance, and the addition of serotonin in plant growth medium results in a loss of insect resistance in mutants (Lu et al., 2018). Here, we provide detailed protocols for determination of serotonin in rice plants, and in SSB and BPH insects, together with the methods for feeding SSB and BPH in artificial diets (added with exogenous serotonin).
Materials and Reagents
- Serotonin measurement
- Sep-Pak C18 cartridge (Waters, catalog number: WAT020515)
- 0.20 µm filter (Millex-LG Millipore, catalog number: SLLGH13NL)
- Syringe (10 ml, Aladdin, A2292-05)
- Syringe (1 ml, Aladdin, A2292-01)
- 50 ml tubes
- 2 ml centrifuge tubes
- 1.5 ml centrifuge tubes
- Rice (Oryza sativa L.) plants, grown in indoor facilities or in the field
Note: In this experiment, the wild type (WT) cultivar Jiazhe B and its OsT5H (encoding Tryptamine 5 hydrolyase) mutant line Jiazhe LM (Lu et al., 2016) were used. - Brown planthopper (BPH; Nilaparvata lugens Stål), raised in laboratory by feeding on one-month-old TN1 (a susceptible rice variety) plants (originally collected from the field)
- Striped stem borer (SSB, Chilo suppressalis Walker), raised in laboratory by feeding on artificial diet
- Liquid Nitrogen
- Methanol (Aladdin, catalog number: M117118)
- Trifluoroacetic acid (Aladdin, catalog number: T103297)
- Perchloric acid (Aladdin, catalog number: P112070)
- Serotonin standard (Sigma, catalog number:14927)
- Ammonium acetate
- Formic acid
- Nitrogen
- Argon
- Assay of BPH survival rate by artificial diet feeding
- Glass cylinder (Figures 2A-2B), home-made, ø, 4 cm; height, 8 cm; with 48 small holes (ø, 0.8 mm)
- Parafilm M (Parafilm, PM996)
- 1.5 ml tubes (centrifuge tubes)
- Filter paper (Sangon Biotech, catalog number: F503316)
- Plastic tube (24 x 95 mm)
- Glycine (Sangon Biotech, catalog number: A100167)
- L-Alanine (Sangon Biotech, catalog number: A600022)
- L-Arginine hydrochloride (Sangon Biotech, catalog number: A600205)
- L-Asparagine (Sangon Biotech, catalog number: A694341)
- L-Aspartic acid (Sangon Biotech, catalog number: A600091)
- L-Cysteine (Sangon Biotech, catalog number: A600132)
- γ-Amino butyric acid (Sangon Biotech, catalog number: A600040)
- L-Glutamic acid (Sangon Biotech, catalog number: A600221)
- L-Glutamine (Sangon Biotech, catalog number: A100374)
- L-Histidine (Sangon Biotech, catalog number: A604351)
- L-Methionine (Sangon Biotech, catalog number: A100801)
- L-Isoleucine (Sangon Biotech, catalog number: A100803)
- L-Leucine (Sangon Biotech, catalog number: A100811)
- L-Proline (Sangon Biotech, catalog number: A600923)
- L-Lysine hydrochloride (Sangon Biotech, catalog number: A110437)
- L-Phenylalanine (Sangon Biotech, catalog number: A600991)
- L-Serine (Sangon Biotech, catalog number: A601479)
- L-Threonine (Sangon Biotech, catalog number: A610919)
- L-Tryptophan (Sangon Biotech, catalog number: A601911)
- L-Tyrosine (Sangon Biotech, catalog number: A601932)
- L-Valine (Sangon Biotech, catalog number: A600172)
- Biotin (Sangon Biotech, catalog number: A100340)
- Calcium pantothenate (Sangon Biotech, catalog number: A600683)
- Choline chloride (Sangon Biotech, catalog number: A600299)
- Folic acid (Sangon Biotech, catalog number: A610466)
- Inositol (Sangon Biotech, catalog number: A600536)
- Nicotinic acid (Sangon Biotech, catalog number: A610660)
- Pyridoxine hydrochloride (Sangon Biotech, catalog number: A600797)
- Riboflavin (Sangon Biotech, catalog number: A600470)
- Thiamine hydrochloride (Sangon Biotech, catalog number: A500986)
- Ascorbic acid (Sangon Biotech, catalog number: A100143)
- CaCl2•2H2O (Sangon Biotech, catalog number: A100556)
- CuCl2•2H2O (Sangon Biotech, catalog number: A603090)
- FeCl3•6H2O (Sangon Biotech, catalog number: A600201)
- MnCl2•4H2O (Sangon Biotech, catalog number: A500331)
- ZnCl2 (Sangon Biotech, catalog number: A501003)
- MgCl2•6H2O (Sangon Biotech, catalog number: A100288)
- KH2PO4 (Sangon Biotech, catalog number: A100781)
- Sucrose (Sangon Biotech, catalog number: A100335)
- BPH artificial diet (D-97) (see Recipes)
- Assay of SSB body weight by artificial diet feeding
- Plastic barrel
- Fresh wild rice (Zizania aquatica) stem (From supermarket) (Han et al., 2012)
- Soybean flour (From supermarket, a China brand)
- Yeast extract powder (From supermarket, a China brand)
- Casein (Sigma, catalog number: C7078)
- Sucrose (Sinopharm Chemical Reagent Co., Ltd., catalog number: A100335)
- Ascorbic acid (Sangon Biotech, catalog number: A100143)
- Sorbic acid (Sangon Biotech, catalog number: SB0899)
- Cholesterol (Sangon Biotech, catalog number: C0433)
- Choline chloride (AMRESCO, catalog number: 0448)
- Wesson's salt Mixture (Sigma, catalog number: W1374)
- 40% Formaldehyde (Sinopharm Chemical Reagent Co., Ltd., Catalog number: 10010018)
- Methyl parahydroxybenzoats
- Agar (Sinopharm Chemical Reagent Co., Ltd., 10000561)
- Nicotinic acid amide (Sangon Biotech, catalog number: A510659)
- Pyridoxine hydrochloride (Sangon Biotech, catalog number: A600797)
- Riboflavin (Sangon Biotech, catalog number: A600470)
- Thiamine hydrochloride (Sangon Biotech, catalog number: A500986)
- Cyanocoblamine (Fluka, catalog number: 82897)
- Folic acid (Sangon Biotech, catalog number: A610466)
- Calcium pantothenate (Sangon Biotech, catalog number: A600683)
- Biotin (Sangon Biotech, catalog number: A100340)
- Semi-artificial diets recipe for SSB (see Recipes)
Equipment
- Refrigerated shaker (Hualida Co., Ltd., model: HZ-9210K)
- Centrifuge (Eppendorf, model: 5804R)
- Rotary evaporator (Shanghai Yarong Biochemical instrument Co., Ltd., model: RE52CS)
- -20 °C freezer
- High performance liquid chromatography, Agilent 1200 infinity series with diode array detector (DAD) in plant serotonin measurement
- HPLC-MS system, Agilent Technologies 1290 with Agilent 6460 triple mass selective detector under multi reaction monitoring (MRM) working mode in BPH serotonin measurement
- C18 column (Agilent, TC-C18(2), 4.6 x 250 mm, 5 μm) for HPLC
- C18 column (Agilent, Zorbax XDB, 2.1 x 150 mm, 3.5 μm) for HPLC-MS
- Analytical balance with 0.00001 g accuracy
- -80 °C freezer
- 4 °C refrigerator
- Growth chamber
- Blender
- Autoclave
- Crisper
Software
- Variance (ANOVA) program StaView, used for statistical analyses
Procedure
- Quantification of serotonin levels in plants
Levels of serotonin in plants were quantified by HPLC according to Kang et al. (2007) with some modifications.- Grind fresh tissue (of leaf/stem/root) in liquid nitrogen using a pestle and immediately store in a -80 °C freezer.
- Weight one to three grams powder and dissolve in 8 ml methanol by shaking for 10 min on an orbital shaker (4 °C, 200 rpm).
- Centrifuge the mixed solution at 13,500 x g for 5 min at 4 °C.
- Transfer and filter the supernatant to a new tube using a syringe with a Millex-LG filter (0.2 μm), Add 2 ml distilled water (1/4 volume of the total) at room temperature.
- Slowly inject the above solution into a pre-activated Sep-pak C18 cartridge (first wash with methanol in three times volume of the cartridge column, followed by distilled water of the same amount) and collect effluent into a 50 ml tube.
- Wash the cartridge using 10 ml 80% methanol and collect the effluent into the 50 ml tube.
- Transfer the effluent into a round-bottomed flask for drying in a rotary evaporator (40 °C under vacuum).
- Add 500 μl 50% methanol into the flask and dissolve the residue by slightly shaking.
- Analyze the serotonin level on reverse-phase HPLC. Inject 10 μl sample solution into a C18 column, mixed with an isocratic solution of 10% methanol in water containing 0.3% trifluoroacetic acid, at a flow rate of 0.8 ml/min, and detect the signal at 280 nm.
- Prepare standard serotonin at concentrations of 2, 1, 0.5, 0.2, 0.1 μg/ml. Analyze 10 μl of serotonin standard solution as above to determine the retention time and quantitative relationship between concentration and peak area.
- Calculate the serotonin concentration according to serotonin retention time point.
- Quantification of serotonin levels in brown planthopper (BPH) (Figure 1)
The content of serotonin in BPH body is lower compared with that in plants, hence the measurement of serotonin needs to be performed on HPLC-MS, here is the protocol modified from Ma et al. (2011).- Collect about 15 adult insects from rice plants into 2 ml centrifuge tubes. After starving for 12 h, freeze in liquid nitrogen, homogenize into powder and store in a -80 ° C freezer.
- Transfer 10-20 mg insect homogenate to 1.5 ml centrifuge tubes, add 300 μl ice-cold 0.1 M perchloric acid, after brief vortexing, and keep on ice for 10 min.
- Centrifuge the mixture at 14,000 x g for 10 min at 4 °C.
- Transfer and filter the supernatant to a new tube using a syringe with a Millex-LG filter (0.2 μm), and store at -20 °C until HPLC-MS analysis.
- Analyze the serotonin level on HPLC-MS system, Agilent Technologies 1290 with Agilent 6460 triple mass selective detector under multi reaction monitoring (MRM) working mode. Inject 10 μl sample solution into a C18 column (Zorbax XDB, 2.1 x 150 mm, 3.5 μm). Mix with a gradient elution at a flow rate of 0.3 ml/min. The elution is composed of 2 mmol/L ammonium acetate containing 0.1% formic acid and methanol with increasing methanol proportion from 19% to 90% (v/v) during the first 2.5 min, and remain the methanol proportion at 90% from 2.5 to 4.5 min.
- Settings for the MS system are: gas temperature: 325 °C, gas flow: 5 L/min, sheath gas temperature: 350 °C, sheath gas flow: 11 L/min, nebulizer pressure: 344.8 kPa, capillary voltage: 3,000 V, nozzle voltage: 500 V in positive mode. Parent ion (m/z) 177, daughter ion (m/z) 160. Use nitrogen as the drying and sheath gas, and argon as collision gas.
- Prepare standard serotonin solution at concentrations of 2, 1, 0.5 ng/ml. Analyze 10 μl of serotonin standard solution as above.
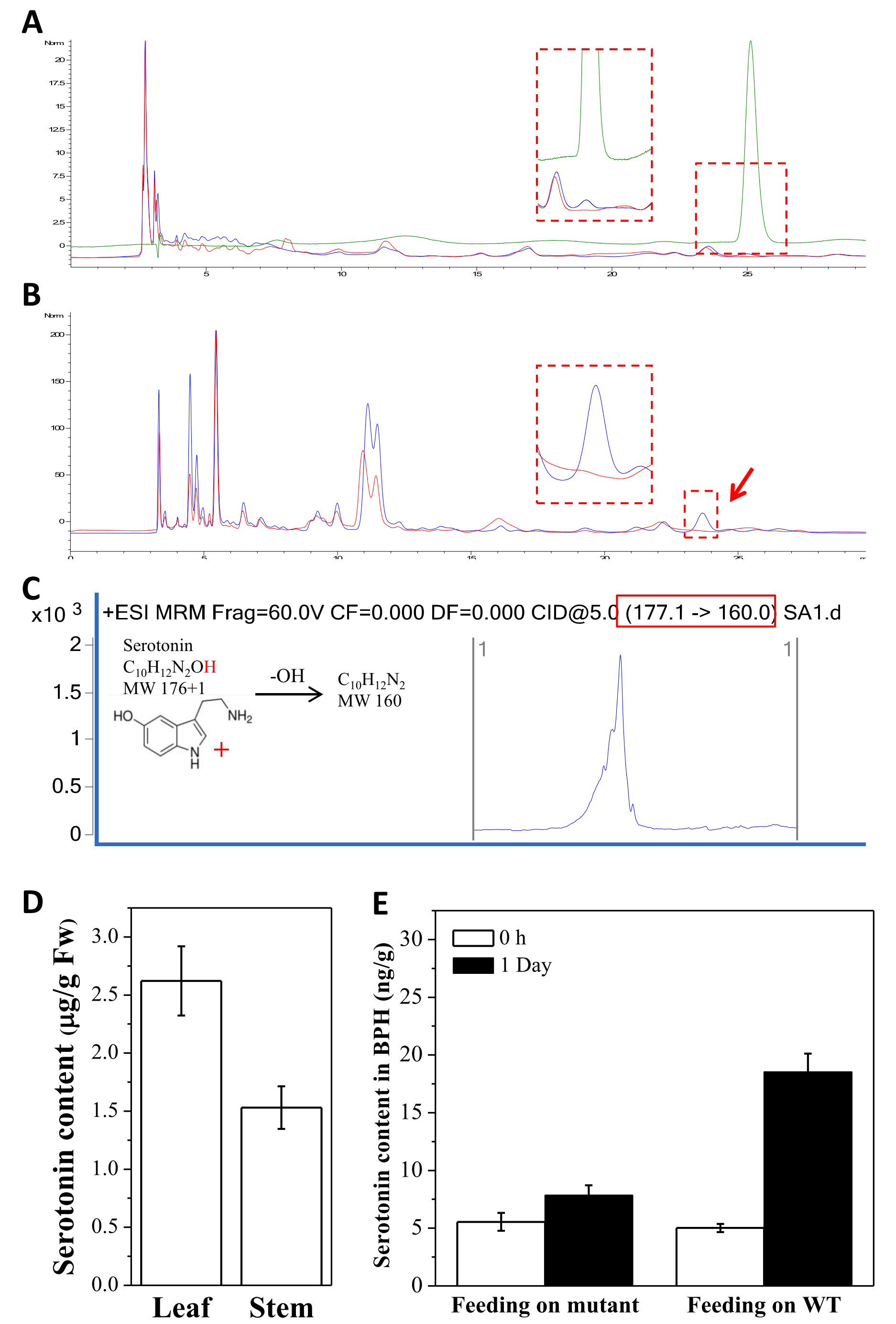
Figure 1. Chromatogram of serotonin measurement (A-C) and serotonin content in rice tissues (D) and in BPH female adults feeding on either WT or mutant rice lines. A. HPLC chromatogram of serotonin, standard (green line), the WT (Jiazhe B, blue line) and its OsT5H mutant. B. HPLC chromatogram of serotonin in WT (blue line) and the mutant (red line). The peak pointed by the red arrow means that the compound serotonin is deficient in the mutants. C. Serotonin measurement using HPLC-MS in multi reaction monitoring (MRM) working mode. D. Serotonin content in leaf and stem of WT Jiazhe B. E. Serotonin content in BPH females after feeding on WT (Jiazhe B) or mutant (Jiazhe LM) rice plants. Prior to the assay, female adults were starved for 12 h.
- BPH artificial diet experiment
The artificial diet experiment was performed as previously described (Fu et al., 2001; Ji et al., 2017).- Prepare artificial diet (D-97) for BPH according to Koyama et al. (1988), modified by Fu et al. (2001), supplemented with serotonin at concentrations of 0.1 and 1 μg/ml.
- Cover one end of the glass cylinder (Figures 2A-2B) with parafilm, drop 40 µl artificial diet (Figure 2C) on to the parafilm at the center, cover with another layer of parafilm (Figures 2D-2F).
- Insert a piece of wet filter paper (2 x 6 cm) and release 20 second-instar BPH nymphs into the cylinder (Figure 2G).
- Cover the other end as Step C2. Wrap the cylinder with a black cotton cloth (Figure 2H).
- Place the cylinders in a growth chamber at 28 ° C under 16 h/8 h day/night cycles with a photon flux density of 250 μmol of photons m-2 s-1.
- Every day, transfer survived nymphs to a new cylinder (prepared the same as above) with fresh diets, and record the mortality at the same time for five days.
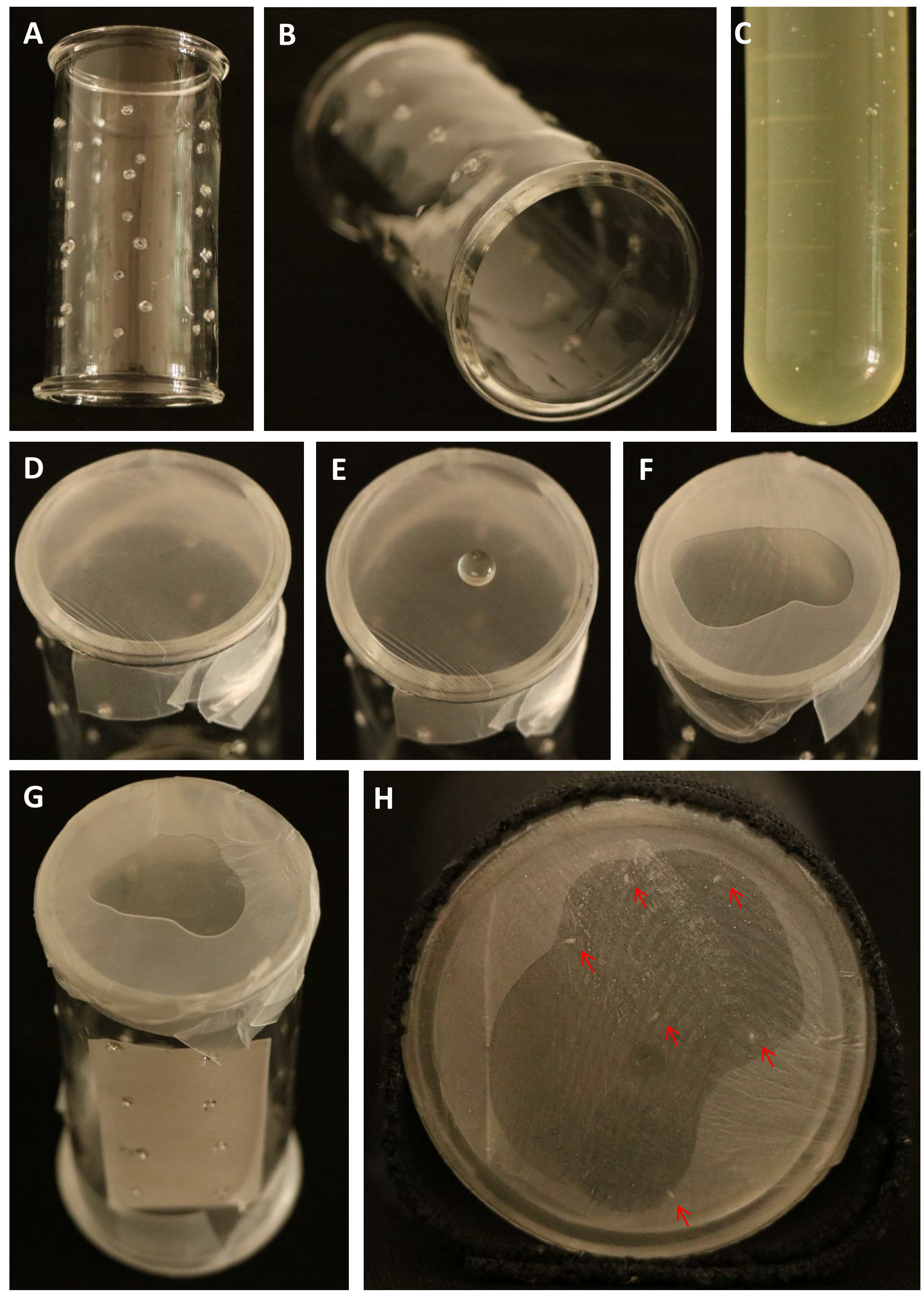
Figure 2. Step-by-step procedure for preparation of cylinders for BPH artificial diet experiment
- SSB artificial diet experiment
- Prepare an artificial diet according to Han et al. (2012), with supplementation of 1 μg/g serotonin as appropriate.
- Cut artificial diet into small patches (Figure 3A) and insert one into each plastic tube (24 x 95 mm).
- For production of neonates, thread 2-3 leaf segments with SSB eggs in one insect pin and place in the tube with artificial diets without serotonin supplementation (Figure 3B).
- Transfer one neonate into one tube with one pellet of the artificial diet supplemented with 1 µg/g serotonin (Figure 3C).
- Place the tubes in a growth chamber with a photon flux density of 250 μM of photos m-2 s-1 in an 8 h light/16 h dark regime at 28 °C. Replace diet every two days (Figure 3D)
- Examine the larvae development stage every day, and weight larvae after each molting until the 6th instar stage.
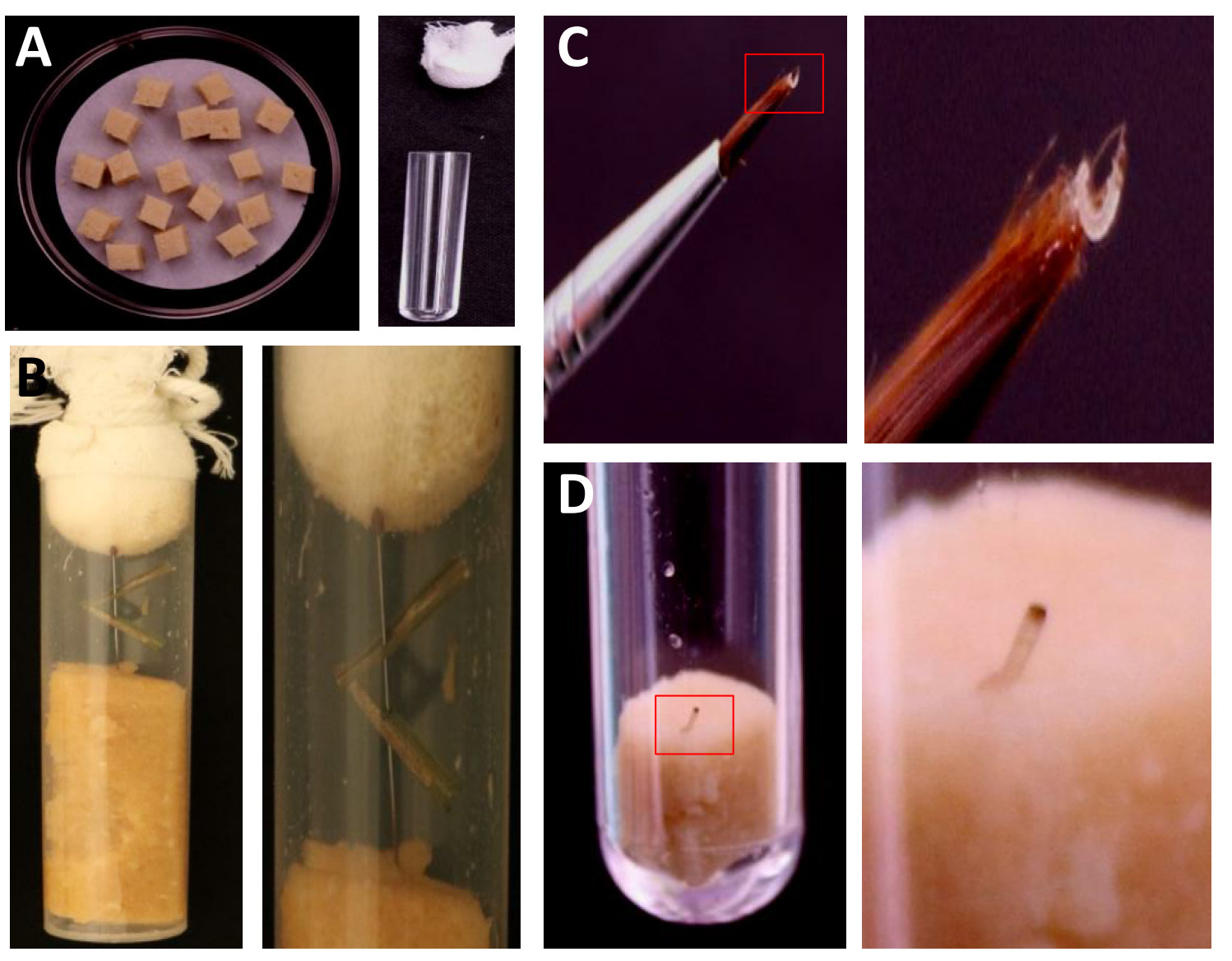
Figure 3. Step-by-step procedure for SSB egg hatching and artificial diet experiment
In support of the role of serotonin in BPH and SSB resistance in rice, the beneficial effect of this hormone was demonstrated in artificial diets, with increased performance of both BPH (Figure 4A) and SSB (Figure 4B).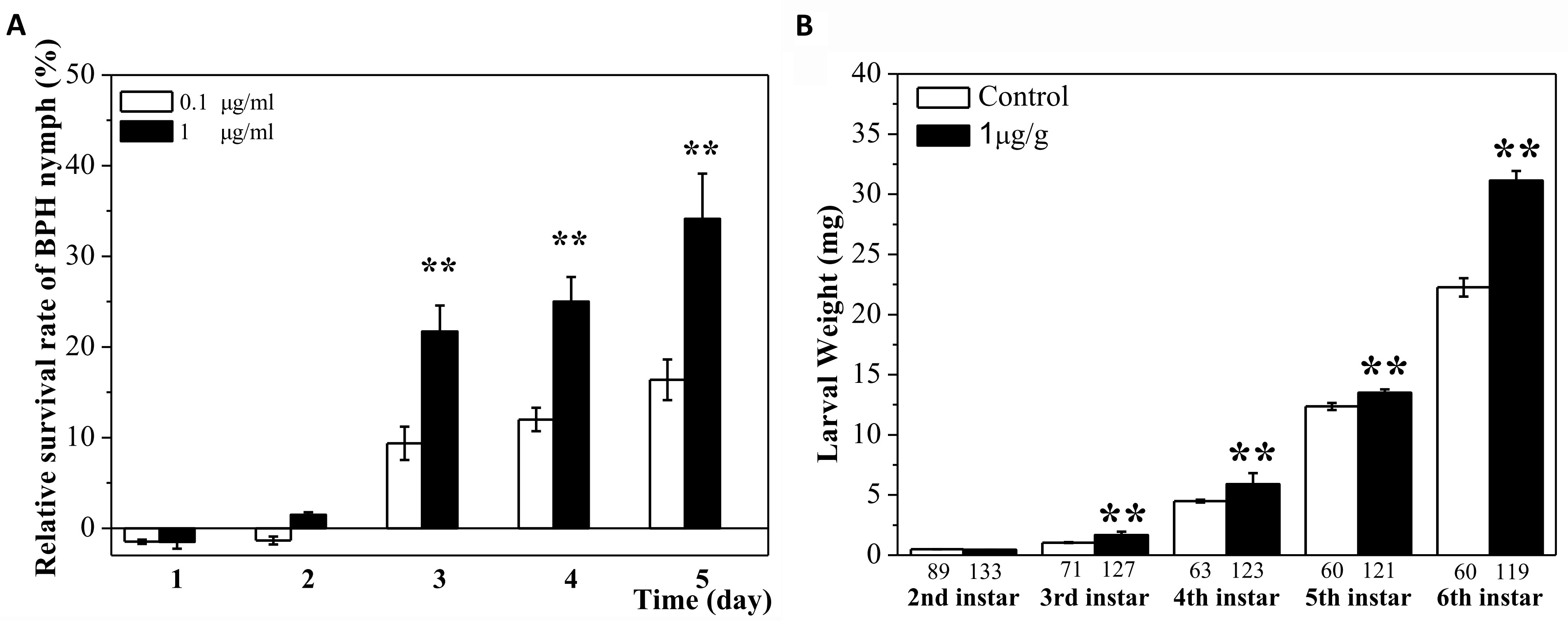
Figure 4. Addition of exogenous serotonin attracts BPH and SSB feeding. A. Addition of serotonin to the artificial diet increases BPH survival rates. Relative survival rate (%) = (Nt-Nc)/Nc x 100, Nc and Nt stand for the number of survived nymphs feeding on an artificial diet without and with serotonin, respectively, at a given time point. B. Addition of serotonin in artificial diet enhances SSB performance. Control stands for artificial diet without serotonin. One hundred SSB larvae were used per treatment.
Data analysis
Statistical analyses were performed using the one-way analysis of variance (ANOVA) programme StaView in these experiments. Data are present in mean values with standard errors as error bars. The differences were considered to be significant when the probability (P) was less than 0.05 in Tukey test.
Notes
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Recipes
- BPH artificial diet (D-97)
For preparation of 100 ml diet, add (mg):
Add distilled water to a total volume of 100 mlGlycine 30 L-Alanine 130 L-Arginine hydrochloride 175 L-Asparagine 230 L-Aspartic acid 100 L-Cysteine 80 L-Cystine hydrochloride 20 γ-Amino butyric acid 10 L-Glutamic acid 250 L-Glutamine 240 L-Histidine 80 L-Methionine 70 L-Isoleucine 100 L-Leucine 240 L-Proline 100 L-Lysine hydrochloride 200 L-Phenylalanine 200 L-Serine 400 L-Threonine 130 L-Tryptophane 105 L-Tyrosine 10 L-Valine 300 Biotin 0.05 Calcium pantothenate 5.0 Choline chloride 50.0 Folic acid 0.5 Inositol 50.0 Nicotinic acid 15.0 Pyridoxine hydrochloride 2.5 Riboflavin 0.5 Thiamine hydrochloride 2.5 Ascorbic acid 100.0 CaCl2•2H2O 3.115 CuCl2•2H2O 0.268 FeCl3•6H2O 2.228 MnCl2•4H2O 0.793 ZnCl2 0.396 MgCl2•6H2O 200 KH2PO4 500 Sucrose 9,000
Adjust the pH to 6.8 - Semi-artificial diets recipe for SSB (5 L)
- Cut 1,000 g fresh Zizania aquatica into patches and homogenize in 2,300 ml water in a blender
- Mix the homogenate with 300 g soybean flour, 200 g yeast extract powder, 100 g casein and 100 g sucrose in a plastic barrel (10 L). Sterilize the mixture under 121 °C for 30 min in an autoclave
- Heat 120 g agar in 2,500 ml water until boiling, pour the above autoclaved mixture into the agar solution
- Prepare 200 ml solution with vitamin C 30 g, sorbic acid 10 g, cholesterol 2 g, choline chloride 3 g, Wesson's salt mixture 1 g, methyl parahydroxybenzoats 10 g and vitamin complex 1 package (containing nicotinic acid amide 0.05216 g; vitamin B1: 0.01312 g; vitamin B2:0.02624 g; vitamin B6: 0.01312 g; vitamin B12: 0.00032 g; folic acid: 0.01312 g; calcium pantothenate: 0.05216 g; biotin: 0.00128 g)
- Pour the 200 ml solution into the Zizania aquatica mixture when it is cooled down to 60 °C, and add 6 ml 40% formaldehyde
- After blended well, put the mixture into crispers and store at 4 °C
Acknowledgments
This study was supported by grants from National Key Research and Development Program of China (2016YFD0102103), Zhejiang Provincial S & T Project on Breeding of Agricultural (Food) Crops (2016C02050-2), China Postdoctoral Research Project (2017M620248).
Competing interests
There are no any conflicts of interest or competing interests.
References
- Fu, Q., Zhang, Z., Hu, C., Lai, F. and Sun, Z. (2001). A chemically defined diet enables continuous rearing of the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Appl Entomol Zool 36 (1): 111-116.
- Han, L., Li, S., Liu P., Peng Y. and Hou, M. (2012). New artificial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann Entomol Soc America 105(2): 253-258.
- Ji, R., Ye, W., Chen, H., Zeng, J., Li, H., Yu, H., Li, J. and Lou, Y. (2017). A salivary Endo-β-1,4-glucanase acts as an effector that enables the Brown Planthopper to feed on rice. Plant Physiol 173(3): 1920-1932.
- Kang, S., Kang, K., Lee, K. and Back, K. (2007). Characterization of tryptamine 5-hydroxylase and serotonin synthesis in rice plants. Plant Cell Rep 26(11): 2009-2015.
- Koyama, K. (1988). Artificial rearing and nutritional physiology of the planthoppers and leafhoppers (Homoptera: Delphacidae and Deltocephalidae) on a holidic diet. JARQ 22(1): 20-27.
- Lu, H. P., Luo, T., Fu, H. W., Wang, L., Tan, Y. Y., Huang, J. Z., Wang, Q., Ye, G. Y., Gatehouse, A. M. R., Lou, Y. G. and Shu, Q. Y. (2018). Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat Plants 4(6): 338-344.
- Lu, H. P., Zhang, H. L., Fu, H. W., Li, Y. F. and Shu, Q. Y. (2016). Identification and characterization of a novel lesion mimic mutant in rice. Journal of Nuclear Agriculture Science 30: 1037-1044. (In Chinese)
- Ma, Z., Guo, W., Guo, X., Wang, X. and Kang, L. (2011). Modulation of behavioral phase changes of the migratory locust by the catecholamine metabolic pathway. Proc Natl Acad Sci U S A 108(10): 3882-3887.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, L., Guo, X., Huang, W., Lu, H. and Shu, Q. (2019). Quantification of Serotonin in Rice and Insect Pest and its Functional Analysis in Insects Using Artificial Diet Feeding. Bio-protocol 9(4): e3173. DOI: 10.21769/BioProtoc.3173.
Category
Plant Science > Plant immunity > Plant-insect interaction
Biochemistry > Other compound > Serotonin
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









