- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Adoptive Transfer of Monocytes Sorted from Bone Marrow
Published: Vol 9, Iss 1, Jan 5, 2019 DOI: 10.21769/BioProtoc.3134 Views: 9068
Reviewed by: Alka MehraAmriti Rajender LullaJia Li

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Macrophage Polarization by Tumor-induced MDSCs Assay
Felipe Vences-Catalán [...] Shoshana Levy
Aug 20, 2016 14259 Views

A 3D Skin Melanoma Spheroid-Based Model to Assess Tumor-Immune Cell Interactions
Marek Wagner and Shigeo Koyasu
Dec 5, 2020 4583 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views
Abstract
Inflammatory Ly6Chi monocytes can give rise to distinct mononuclear myeloid cells in the tumor microenvironment, such as monocytic myeloid-derived suppressor cells (Mo-MDSC), immature macrophages, M2-like tumor-associated macrophages (TAMs), M1-like TAMs or monocyte-derived dendritic cells (Mo-DCs). This protocol describes a method to assess the fate and recruitment of inflammatory Ly6Chi monocytes in the tumor microenvironment.
Keywords: Adoptive transferBackground
Tumors are heterogeneous microenvironments where complex interactions take place between neoplastic cells and infiltrating inflammatory cells, such as tumor-associated macrophages (TAMs) and tumor-associated dendritic cells (TADCs). The relevance of tumor-infiltrating mononuclear myeloid cells is underscored by clinical studies showing a correlation between their abundance and poor prognosis (Bolli et al., 2007). The origin of TAMs and TADCs has been a matter of debate, since several levels of complexity result in considerable TAM and TADC heterogeneity (Movahedi et al., 2010; Laoui et al., 2014, Laoui et al., 2016; Van Overmeire et al., 2016; Kiss et al., 2018). Here, we describe a valuable method to adoptively transfer bone-marrow derived monocytes permitting the assessment of their recruitment and fate in tumors.
Materials and Reagents
- Polyester filters cut in 10 x 10 cm squares, thread diameter 70 μm (Specturmlabs, catalog number: 146490)
- 10 ml syringes (Omnifix, catalog number: 473203)
- 1 ml syringes (Greiner, catalog number: 470203)
- 27 G needles (BD Bioscience, catalog number: 300635)
- 25 G needles (BD Biosciences, catalog number: 300400)
- 19 G needles (BD Biosciences, catalog number: 301500)
- Falcon standard tissue culture dish (Fisher Scientific, catalog number: 353003)
- BD Falcon 50 ml polypropylene tubes (BD Biosciences, catalog number: 2070)
- BD Falcon 15 ml polypropylene tubes (BD Biosciences, catalog number: 2096)
- BD Falcon 5 ml polypropylene round-bottom tube (BD Biosciences, catalog number: 352063)
- 70 µm sterile nylon gauze
- LS columns (Miltenyi, catalog number: 130-042-401)
- Naive mice: Age preferably between 6 and 12 weeks, strain can vary depending on the experiment/project (in this example we used C57BL/6 mice)
- Ethanol absolute analaR Normapur ACS (VWR Chemicals, catalog number: 84857360)
- RPMI-1640 medium (RPMI) (Life Technologies, catalog number: 52400-041)
- Fetal calf serum (FCS) (Life Technologies, Gibco, catalog number: DE14-801F)
- L-glutamine (Life Technologies, catalog number: 25030-024)
- Penicillin-streptomycin (Life Technologies, catalog number: 15140-130)
- Ammonium chloride (NH4Cl) (Merck KGaA, catalog number: 1011450500)
- Potassium bicarbonate (KHCO3) (Merck KGaA, catalog number: 104852)
- EDTA (Duchefa Biochemie, catalog number: E0511.1000)
- Hank’s buffered salt solution (HBSS) (Life Technologies, Gibco, catalog number: 14175129)
- Anti-CD11b microbeads (Miltenyi, catalog number: 130-049-601)
- Purified CD16/CD32 (FcBlock) (clone 2.4G2) (BD Biosciences, catalog number: 553142)
- PE-Cy7-conjugated anti-CD11b antibody (clone M1/70) (BD Biosciences, catalog number: 552850)
- AF647-conjugated anti-Ly6C antibody (clone ER-MP20) (Serotec, catalog number: MCA2389A647)
- PerCP-Cy5.5-conjugated anti-I-A/I-E (MHC-II) antibody (clone M5/114.15.2) (BioLegend, catalog number: 107626)
- FITC-conjugated anti-Ly6G antibody (clone 1A8) (BD Biosciences, catalog number: 551460)
- APC-Cy7-conjugated anti CD45 (clone 30-F11) (BioLegend, catalog number: 103116)
- CellTrace Violet (Thermo Fisher Scientific, Molecular probesTM, catalog number: C34557)
- Trypan blue (Life Technologies, Gibco, catalog number: 15250061)
- DMSO
- 70% ethanol (see Recipes)
- Complete medium (see Recipes)
- Erythrocyte lysis buffer (see Recipes)
- MACS buffer (see Recipes)
- Sorting buffer (see Recipes)
- Violet tracer (see Recipes)
Equipment
- Sterile culture hood, PSM Optimale 18 (ADS)
- Surgical scissors and forceps
- 37 °C, 5% CO2 cell culture incubator (Binder, VWR)
- Pipettes (Gilson)
- Centrifuges 5810 R (Eppendorf, model: 5810 R)
- Shaker KS 260 Basic (IKA, model: KS 260 basic)
- Microscope Eclipse TS100 (Nikon, model: Eclipse TS100)
- MidiMACSTM Separator and MultiStand (Miltenyi, catalog number: 130-042-301)
- Multicolor FACS Sorter-FACS Aria II (BD Biosciences Aria flow cytometer)
Procedure
- Preparation of a bone-marrow single cell suspension
- Sacrifice a naive mouse and restrain it by pinning its paws into a foam surface using syringe needles. Disinfect the skin of the mouse with 70% ethanol (see Recipe 1). Make a parallel incision from the base of the tail up to the neck along the mouse’s abdomen and to the paws without puncturing the peritoneum. Gently pull back the skin and pin it to the foam surface to expose the hind limb (Figure 1A).
- Cut the hind limb free from the skin and the body by cutting in the pelvis just behind the femur-pelvis joint. Keep the femur and tibia whole. Try to remove as much excess tissue (muscles, fibers...) surrounding the bone as possible using scissors or with a scalpel (Figure 1B). Do this procedure gently in order to avoid breakage of the bones.
- Gently pull the hind paw from the limb by moving it back and forwards (Figure 1C).
- Clean the bone by submerging it in 70% ethanol and store the bone in 5 ml complete medium (see Recipe 2) in a 50 ml Falcon tube on ice.
- Repeat this action with the second hind limb.
- Detach the tibia from the femur and cut the fibula and patella away and put the bones in a Falcon standard tissue culture dish (Figures 1D, 1E and 1F).
- Insert a 27 G needle attached to a 10 ml syringe containing complete medium in the femur and gently flush the femur with 10 ml complete medium (Figures 1G and 1H). Before flushing, the tibia cut the ‘white’ bone that was adjacent to the paw away and insert the needle in the bone at the other side. If any resistance is perceived when flushing the bones, cut a small fraction of the bone from the end and reinsert the needle in the remaining bone. The flushing is complete when all the red bone marrow is in the plate, and red tissue can no longer be seen in the bone.
- Homogenize the bone-marrow by passing (aspirating and pressing) the medium containing bone-marrow two times through the 19 G needle (Figures 1I and 1J).
- Filter the bone-marrow suspensions through a 70 µm sterile nylon gauze into a sterile 50 ml conical tube and wash the culture plate and the gauze with 10 ml complete medium.
- Centrifuge the 50 ml tubes at 450 x g for 6 min at 4 °C and discard the supernatants (Figure 1K).
- Remove the red blood cells by resuspending the pellet in 5 ml erythrocyte lysis buffer (see Recipe 3) and leave at room temperature for 2 min.
- Neutralize by adding 15 ml complete medium, and transfer the suspension to a new 50 ml tube through a 70 µm sterile nylon gauze.
- Centrifuge the 50 ml tubes at 450 x g for 6 min at 4 °C and discard the supernatants (Figure 1L).
- Count the living cells using trypan blue and resuspend the cells in MACS buffer (see Recipe 4) at a concentration of 108 cells/ml. From the total bone marrow, in general, 10% to 15 % normally would constitute monocytes (varies slightly with age, mouse strain, and animal house type), which can be enriched/purified as explained below.
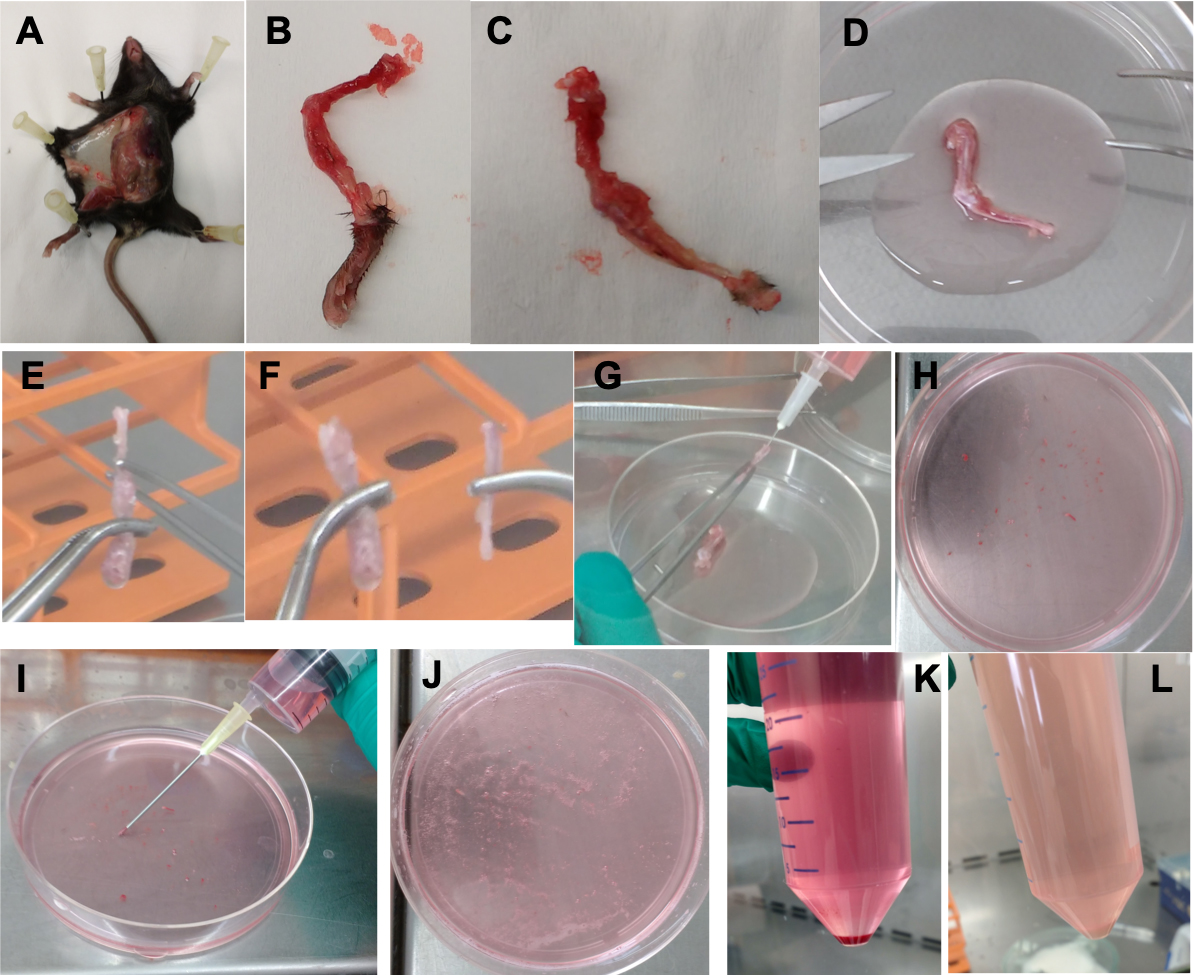
Figure 1. Bone-marrow single-cell preparation. A. Naive mouse. B. Hind limb with paw. C. Hind limb without paw. D. Cleaned hind limb without paw. E. Detaching of the tibia from the femur. F. Femur (left) and tibia (right). G. Flushing the bones. H. Bone marrow right after flushing. I. Homogenization of the bone marrow. J. Homogenized bone-marrow single cell suspension. K. Cell pellet before erythrocyte lysis buffer. L. Cell pellet after erythrocyte lysis buffer.
- Purification of Ly6Chigh monocytes from the bone marrow
- Add a 5 µl aliquot of anti-CD11b magnetic microbeads per 107 cells and incubate for 20 min at 4 °C on an orbital shaker at 50 rpm.
- Wash by adding 10 ml MACS buffer, centrifuge at 450 x g for 6 min at 4 °C and discard the supernatants.
- Place an LS column in a MidiMACSTM Separator attached to a magnetic MultiStand and wash it by putting 3 ml MACS buffer on the top. The liquid passes the column by gravity.
- Resuspend the pelleted cells in 1 ml MACS buffer and pipette the labeled cell suspension on top of the LS separation column. When the cell suspension has passed through the column, wash the column by adding 3 x 3 ml MACS buffer.
- Remove the LS column from the separator, add 5 ml MACS buffer on top of the column and immediately flush the column by firmly pressing the provided plunger on the column to wash the magnetically labeled cells out in a sterile 15 ml tube.
- Incubate the CD11b+ cell suspension with rat anti-mouse CD16/CD32 (10 µg per 107 cells) on ice-cold water for 20 min, in order to block the Fc receptors present on the cells’ surface.
- Incubate the cell suspension with fluorescently labeled antibodies of interest (1 µg per 107 cells) for another 20 min on ice-cold water, protected from exposure to light.
- Wash by adding 10 ml MACS buffer, centrifuge at 450 x g for 6 min at 4 °C and discard the supernatants.
- Meanwhile, precoat 5 ml polypropylene round-bottom tubes and 15 ml tubes with heat-inactivated fetal calf serum, add respectively 1 ml or 2 ml heat-inactivated fetal calf serum. Shake the tubes gently by hand so that the heat-inactivated fetal calf serum covers the whole surface of the tube, and discard the excess of heat-inactivated fetal calf serum. This will prevent the cells to stick to the tubes and hence enhance the recovery of cells.
- Resuspend the pellet in 1 ml sorting buffer (see Recipe 5) per 107 cells and transfer into a sterile 5 ml polypropylene round-bottom tube precoated with heat-inactivated fetal calf serum.
- Ly6Chigh monocytes can be sorted on a BD FACS Aria as CD45pos CD11bpos Ly6Gneg Ly6Chigh MHC-IIneg cells (Laoui et al., 2016; Van Overmeire et al., 2016).
- Collect the sorted monocytes in 15 ml tubes precoated with heat-inactivated fetal calf serum containing 3 ml complete medium.
- Labeling of the monocytes for in vivo tracking
- Option I: Bone-marrow donor mice are wild-type mice (Laoui et al., 2016). Ideally, the donor and recipient differ in their CD45 allele (CD45.1 vs. CD45.2).
- Centrifuge the 15 ml tubes containing the sorted monocytes at 450 x g for 10 min at 4 °C and discard the supernatant.
- Resuspend the cell pellet in HBSS at a concentration of 106/ml and add 1 μl of CellTrace (see Recipe 6) to stain 1 ml cells (hence a 1:1,000 dilution). It is important that the labeling happens in a protein-free medium. Incubate the cell suspension for 20 min at 37 °C, protected from exposure to light.
- Wash by adding 10 ml HBSS, centrifuge at 450 x g for 6 min at 4 °C and discard the supernatant.
- Resuspend the cell pellet in HBSS at a concentration of 5 x 106/ml and keep on ice till entering the animal facility.
- Inject the recipient CD45.2 mice with 200 μl intravenously in the tail vein using a 25 G needle and a 1 ml syringe (Video 1).Video 1. Intravenously tail vein injection. (This video was made at Vrije Universiteit Brussels according to guidelines from the Belgian Council for Laboratory Animal Sciences and were approved by the Ethical Committee for Animal Experiments of the Vrije universiteit Brussels (license 15-220-2).
- The progeny of the monocytes can be traced as from one day, until 10 days after inoculation (ideally 48 to 72 h). The monocyte-derived cells can be traced in the Pacific Blue-channel (405 nm) by flow cytometry. In addition, CD45.1 and CD45.2 can be used as a complementary staining.
- Option II: Bone-marrow donor mice are Ubiquitin-GFP mice (Van Overmeire et al., 2016).
- Centrifuge the 15 ml tubes containing the sorted monocytes at 450 x g for 10 min at 4 °C and discard the supernatant.
- Resuspend the cell pellet in HBSS at a concentration of 5 x 106/ml and keep on ice till entering the animal facility.
- Inject the recipient CD45.2 mice with 200 μl of cells intravenously in the tail vein using a 25 G needle and a 1 ml syringe (Video 1).
- The progeny of the monocytes can be traced as from one day, until 10 days after inoculation (ideally 48 to 72 h). The monocyte-derived cells can be traced in the FITC-channel by flow cytometry.
- Option I: Bone-marrow donor mice are wild-type mice (Laoui et al., 2016). Ideally, the donor and recipient differ in their CD45 allele (CD45.1 vs. CD45.2).
Data analysis
Flow cytometry is commonly used to visualize the transferred monocytes in the tumors of the recipient mice. As the transferred monocytes are diluted systemically in the recipient mice, only few cells will reach the tumors or other organs of interest and differentiate in situ into macrophages or dendritic cells (Figures 2 and 3). Hence, it is important to acquire many events by flow cytometry. After gating on the transferred CellTrace+ or GFP+ cells, the fate of their progeny can be determined using additional markers specific for monocytic myeloid-derived suppressor cells (Mo-MDSC), immature macrophages, M2-like tumor-associated macrophages (TAM), M1-like TAM or monocyte-derived dendritic cells (Laoui et al., 2016; Van Overmeire et al., 2016). For these type of experiments, two independent repeats containing each n ≥ 3 are generally accepted as many donor mice can be needed for acquiring enough cell in the receptor mice.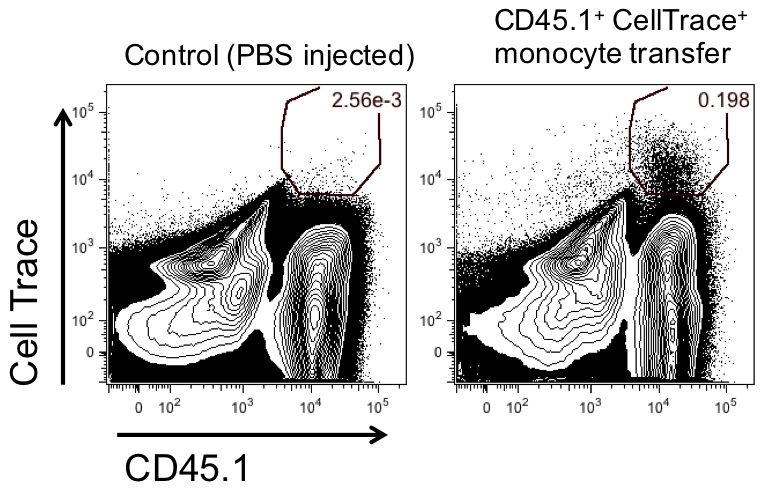
Figure 2. Adoptive transfer of CellTrace-labeled CD45.1+ monocytes in CD45.2 recipient mice. One million CellTrace-labeled CD45.1+ monocytes were adoptively transferred to 11-day old LLC tumor-bearing mice. Two days after CellTrace-labeled CD45.1+ monocytes transfer, mice were sacrificed and tumors were collected. Graphs show the percentage of GFP+Ly6Chi monocytes present in total tumors.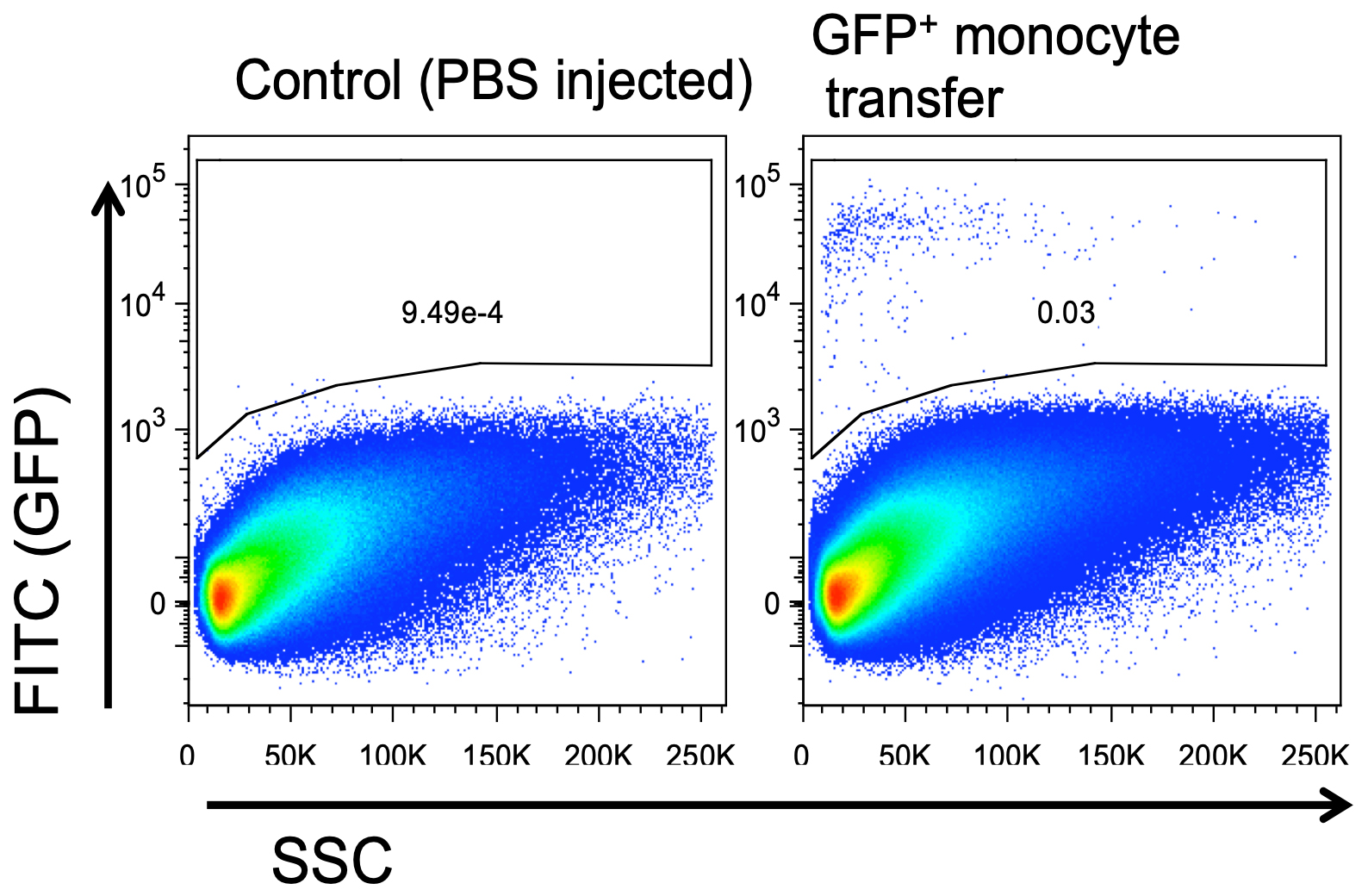
Figure 3. Adoptive transfer of GFP+ monocytes in CD45.2 recipient mice. One million GFP+ monocytes were adoptively transferred to 11-day old LLC tumor-bearing mice. Four hours after GFP+ monocyte transfer, mice were sacrificed, and tumors were collected. Graphs show the percentage of GFP+Ly6Chi monocytes present in total tumors.
Recipes
- 70% ethanol (for 100 ml)
70 ml 99.9% ethanol (VWR Chemicals)
30 ml demineralized water - Complete medium
Roswell Park Memorial Institute (RPMI)-1640
10% (v/v) heat-inactivated fetal calf serum (FCS)
300 μg•ml-1 L-glutamine
100 U•ml-1 penicillin
100 μg•ml-1 streptomycin - Erythrocyte lysis buffer
8.29 g•L-1 NH4Cl
1 g•L-1 KHCO3
37.2 mg•L-1 EDTA
Bring at pH 7.2 - MACS buffer
Hank’s buffered salt solution
0.5% (v/v) heat-inactivated fetal calf serum
2 mM EDTA - Sorting buffer
Hank’s buffered salt solution
0.5% (v/v) heat-inactivated FCS
5 mM EDTA - Violet tracer
Resuspend CellTrace in 20 μl DMSO (provided)
Acknowledgments
The authors thank FWO-Vlaanderen, ‘Stichting tegen Kanker’ and ‘Komop tegen kanker’ for their support. This protocol was adapted from Laoui et al., (2016), Nat Comm, and Van Overmeire et al., (2016), Cancer Res. EVO and CA are supported by PhD grants from the Research Foundation Flanders (FWO). DL is supported by grants from Kom op tegen kanker and Vrije Universiteit Brussel. JVG is supported by grants from Kom op tegen kanker, FWO and Stichting tegen kanker.
Competing interests
The authors declare no competing financial interests.
Ethics
All procedures followed the guidelines of the Belgian Council for Laboratory Animal Science and were approved by the Ethical Committee for Animal Experiments of the Vrije Universiteit Brussel (licenses 11-220-3 and 15-220-2).
References
- Bolli, E., Movahedi, K., Laoui, D. and Van Ginderachter, J. A. (2017). Novel insights in the regulation and function of macrophages in the tumor microenvironment. Curr Opin Oncol 29(1): 55-61.
- Kiss, M., Van Gassen, S., Movahedi, K., Saeys, Y. and Laoui, D. (2018). Myeloid cell heterogeneity in cancer: not a single cell alike. Cell Immunol 330:188-201.
- Laoui, D., Keirsse, J., Morias, Y., Van Overmeire, E., Geeraerts, X., Elkrim, Y., Kiss, M., Bolli, E., Lahmar, Q., Sichien, D., Serneels, J., Scott, C. L., Boon, L., De Baetselier, P., Mazzone, M., Guilliams, M. and Van Ginderachter, J. A. (2016). The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat Commun 7: 13720.
- Laoui, D., Van Overmeire, E., Di Conza, G., Aldeni, C., Keirsse, J., Morias, Y., Movahedi, K., Houbracken, I., Schouppe, E., Elkrim, Y., Karroum, O., Jordan, B., Carmeliet, P., Gysemans, C., De Baetselier, P., Mazzone, M. and Van Ginderachte,r J. A. (2014). Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res 74(1): 24-30.
- Movahedi, K., Laoui, D., Gysemans, C., Baeten, M., Stange, G., Van den Bossche, J., Mack, M., Pipeleers, D., In't Veld, P., De Baetselier, P. and Van Ginderachter, J. A. (2010). Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70(14): 5728-5739.
- Van Overmeire, E., Stijlemans, B., Heymann, F., Keirsse, J., Morias, Y., Elkrim, Y., Brys, L., Abels, C., Lahmar, Q., Ergen, C., Vereecke, L., Tacke, F., De Baetselier, P., Van Ginderachter, J. A. and Laoui, D. (2016). M-CSF and GM-CSF receptor signaling differentially regulate monocyte maturation and macrophage polarization in the tumor microenvironment. Cancer Res 76(1): 35-42.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Laoui, D., Overmeire, E. V., Abels, C., Keirsse, J. and Ginderachter, J. A. V. (2019). Adoptive Transfer of Monocytes Sorted from Bone Marrow. Bio-protocol 9(1): e3134. DOI: 10.21769/BioProtoc.3134.
Category
Immunology > Animal model > Mouse
Cancer Biology > Tumor immunology > Tumor microenvironment > Immunosuppression
Cell Biology > Cell-based analysis > Extracellular microenvironment
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










