- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Maintenance of Schmidtea mediterranea in the Laboratory
Published: Vol 8, Iss 19, Oct 5, 2018 DOI: 10.21769/BioProtoc.3040 Views: 6644
Reviewed by: Ivan ZanoniAchille BroggiMarco Di Gioia

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurement of Respiration Rate in Live Caenorhabditis elegans
Li Fang Ng and Jan Gruber
May 20, 2019 9000 Views

Pea Aphid Rearing, Bacterial Infection and Hemocyte Phagocytosis Assay
Li Ma [...] Zhiqiang Lu
Dec 20, 2020 3432 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 211 Views
Abstract
In the last years, planarians have emerged as a unique model animal for studying regeneration and stem cells biology. Although their remarkable regenerative abilities are known for a long time, only recently the molecular tools to understand the biology of planarian stem cells and the fundamentals of their regenerative process have been established. This boost is due to the availability of a sequenced genome and the development of new technologies, such as interference RNA and next-generation sequencing, which facilitate studies of planarian regeneration at the molecular and genetic level. For these reasons, maintain a healthy and stable planarian population in the laboratory is essential to perform reproducible experiments. Here we detail the protocol used in our laboratory to maintain the planarian species Schmidtea mediterranea, the most widespread as a model.
Keywords: PlanarianBackground
Planarians are bilaterally symmetric platyhelminthes, members of the superphylum lophotrochozoa. There are terrestrial, marine, and freshwater planarians. They prey predominantly upon injured insects, insect larvae, and other invertebrates. Planarians are triploblastic and acoelomated animals that lack circulatory, skeletal, and respiratory systems (Figure 1A). These animals have the amazing ability to restore any missing part of their body after an amputation in a few days (Reddien and Alvarado, 2004; Salo, 2006); and to grow and degrow depending of the environmental conditions and food availability (Baguñá and Romero, 1981). These characteristics are due to the presence of an adult stem cell population – called neoblasts – that is able to give rise to any planarian cell type (Reddien and Alvarado, 2004; Salo, 2006). The high regenerative capacity of planarians, with the presence of a unique totipotent stem cell system, provides an ideal model for studying cell renewal, regeneration, and stem cell regulation. Schmidtea mediterranea is the most common planarian species used in molecular biology to perform molecular and cellular studies, because it presents special features that optimize research. For instances, they are easily maintained as a stable clonal line in the laboratory due to their robust ability to regenerate, which allows a uniform genetic background and minimizes the experimental variability. Here we detail the protocol used in our laboratory to maintain the planarian species Schmidtea mediterranea.
Materials and Reagents
- Glass Tupperware (Figure 1B)
The size may vary depending on the requirements of each experiment. You must avoid large containers that can be too heavy and difficult to manipulate. - Assexual planarian clonal line of the asexual strain of Schmidtea mediterranea
This species can be found in coastal areas in the Western Mediterranean. The origin of the strain used in most labs are the fountains of Montjuic in Barcelona. The easiest source nowadays is asking directly from our laboratory or any that has already stablished it as a model organism. - Organic beef liver
Note: It can be purchased in any butcher shop that sells organic meat. - NaOH (Merck, catalog number: 106462 )
- MgSO4•7H2O (Merck, catalog number: 105886 )
- NaHCO3 (Merck, catalog number: 106329 )
- KCl (Merck, catalog number: 104936 )
- MgCl2•6H2O (Merck, catalog number: 105833 )
- CaCl2•2H2O (Merck, catalog number: 102382 )
- MilliQ water
- 100x Planarians Artificial Medium (PAM) stock solution (see Recipes)
- 100x CaCl2 Stock solution (see Recipes)
Equipment
- "Planarium"–room or incubator at 20 °C (Figure 1C)
- 1 L Beaker
- Magnetic stirrer
- Stir bar
- 1 L Graduated cylinder
- 1 L Crystal bottle
- Water tank

Figure 1. Culture of Schmidtea mediterranea. A. In vivo planarian from the species Schmidtea mediterranea. B. Glass Tupperware with planarians. C. “Planarium” (room at 20 °C).
Procedure
- Prepare 100x Planarians Artificial Medium (PAM) stock solution (see Recipe 1).
Note: Separated stocks of 100x PAM water and 100x CaCl2•2H2O solution should be prepared to avoid salt precipitation. It is not recommended to prepare a higher concentrated stock because salts may precipitate. Dilute the appropriate amount of both solutions and mix them just before their use (see Recipes). Do not store the diluted stocks in the water tanks for more than 1 week. - Prepare 100x CaCl2 Stock solution (see Recipe 2).
- When required, dilute the stocks to 1x in Millipore water using the water tanks. For 10 L of 1x PAM water use 100 ml of each stock.
- Put planarians, obtained from a clonal line strain of Schmidtea mediterranea in a glass Tupperware and fill it with 1x PAM water up to ¾ of the Tupperware capacity (Figure 1B). Keep planarians in a "Planarium": a separated room or incubator with controlled temperature (around 20 °C) and maintained in the dark.
Note: To increase the population, planarians can be cut at postpharyngeal level, which is the region that fissions during natural asexual reproduction. The fragments will completely regenerate the missing parts in two weeks. If planarians are big (> 0.5 cm) and a high increase in the population is desired, an additional cut can be performed prepharyngeally. - To promote planarians grow and reproduction, feed them 2 or 3 times a week with organic beef liver. To do that, immerse small pieces of liver in the water during 5-7 h (a piece of 4 cm2 cut in smaller fragments can be added in a 150 cm2 Tupperware). After this time, remove the liver and change with the PAM water. When mucus is observed in the Tupperware walls, clean it with a piece of paper without touching planarians and add new 1x PAM water.
Notes:- For long-term storage, beef liver should be cut into small pieces (around 3-5 cm), wrapped in foil and frozen. When use it to feed planarians, remove the foil and cut it into small pieces with a scalpel.
- Be aware to cut some openings in the Tupperware cover to allow the gas exchange.
- Normally PAM water must be changed only after feeding. However, when mucus is clearly observed in the Tupperware walls, planarian water should be changed even if they have not been fed. You must be aware to avoid infections.
- To increase the planarian population, planarians can be cut at postpharyngeal level, which is the region that fissions during natural asexual reproduction. The fragments will completely regenerate the missing parts in two weeks. If planarians are big (> 0.5 cm) and a high increase in the population is desired, an additional cut can be performed prepharyngeally. The density of the planarians can be varied according to the specific requirements of each researcher. The culture showed in Figure 1B corresponds to a low-density culture. It can be increased up to 2-3 times more.
Data analysis
To perform experiments, planarians of the same size should be chosen to avoid results discrepancy due to differences in size. For the same reason, only fully regenerated planarians should be used (see Figure 2).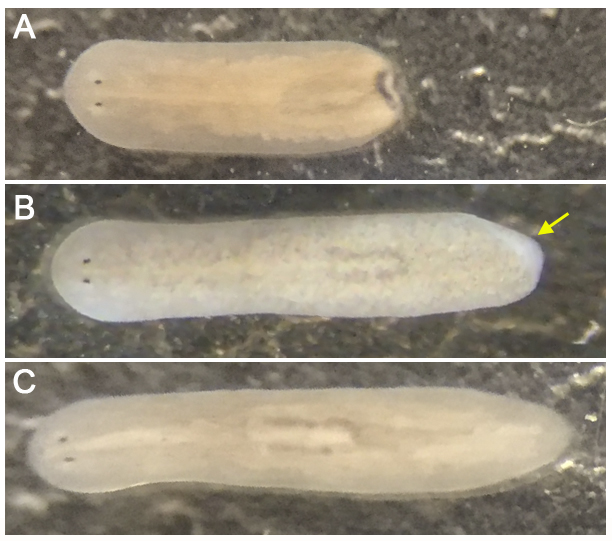
Figure 2. Tail regeneration of Schmidtea mediterranea. A. Planarian that has been just cut tail. B. Planarian that has partially regenerated the tail (see white blastema pointed with a yellow arrow), 6-8 days after the cut. C. Fully regenerated planarian, 10-13 days after the cut.
Recipes
- 100x Planarians Artificial Medium (PAM) stock solution
1.6 mM NaCl
1.0 mM MgSO4•7H2O
1.2 mM NaHCO3
0.1 mM KCl
0.1 mM MgCl2•6H2O- Add around 750 ml of MilliQ water to a beaker
- Put the beaker in the magnetic stirrer with a stir bar
- Add the salts one by one to the MilliQ water to reach the appropriated concentration. Before adding the next salt, confirm that the previous one is totally dissolved
- Transfer the solution to 1 L graduated cylinder and adjust the volume to 1 L
- Store this stock in a 1 L glass bottle in the “Planarium”
- 100x CaCl2 Stock solution
1.0 mM CaCl2•2H2O- Add around 750 ml of MilliQ water to a beaker
- Put the beaker in the magnetic stirrer with a stir bar
- Add the salt to the MilliQ water to reach the appropriated concentration
- Transfer the solution to 1 L graduated cylinder and adjust the volume to 1 L
- Store this stock in a 1 L glass bottle in the “Planarium”
- PAM water
0.016 mM NaCl
0.01 mM MgSO4•7H2O
0.012 mM NaHCO3
0.001 mM KCl
0.001 mM MgCl2•6H2O
0.01 mM CaCl2•2H2O
Note: PAM water must be prepared from two different 100x stock solutions (see Recipes 1 and 2), since the CaCl2 must be added only when required, to avoid precipitation.
Acknowledgments
We acknowledge Eudald Pascual for the images in Figure 2. This work was supported by grant BFU2008-01544 and BFU2014-56055-P (Ministerio de Educación y Ciencia) and grant 2009SGR1018 (AGAUR). N.S. was supported by the APIF fellowship from the Universitat de Barcelona. This work is adapted from previous literature (Cebrià and Newmark, 2005).
Competing interests
The authors declare no conflicts of interest or competing interests.
References
- Baguñá, J. and Romero, R. (1981). Quantitative analysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia 84(1): 181-194.
- Cebrià, F. and Newmark, P. A. (2005). Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 132(16): 3691-3703.
- Reddien, P. W. and Alvarado, A. S. (2004). Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol 20: 725-757.
- Salo, E. (2006). The power of regeneration and the stem-cell kingdom: freshwater planarians (Platyhelminthes). Bioessays 28(5): 546-559.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sousa, N. D. and Adell, T. (2018). Maintenance of Schmidtea mediterranea in the Laboratory. Bio-protocol 8(19): e3040. DOI: 10.21769/BioProtoc.3040.
Category
Cell Biology > Model organism culture > Maintenance
Stem Cell > Adult stem cell > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link