- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessing Classical Olfactory Fear Conditioning by Behavioral Freezing in Mice
Published: Vol 8, Iss 18, Sep 20, 2018 DOI: 10.21769/BioProtoc.3013 Views: 6232
Reviewed by: Andrew L. EagleArnau Busquets-GarciaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Characterizing Hedonic Responses to Flavors Paired with Internal Pain and Nausea through the Taste Reactivity Test in Rats
Matías López [...] Azucena Begega
Sep 20, 2022 2184 Views

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1660 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2628 Views
Abstract
Classical fear conditioning typically involves pairing a discrete cue with a foot shock. Quantifying behavioral freezing to the learned cue is a crucial assay for neuroscience studies focused on learning and memory. Many paradigms utilize discrete stimuli such as tones; however, given mice are odor-driven animals and the wide variety of odorants commercially available, using odors as conditioned stimuli presents advantages for studies involving learning. Here, we describe detailed procedures for assembling systems for presenting discrete odor cues during single-day fear conditioning and subsequent analysis of freezing behavior to assess learning.
Keywords: Classical conditioningBackground
Associative fear learning, the root of several anxiety disorders, involves pairing a neutral stimulus with an aversive outcome. This pairing produces robust behavioral fear responses, in the form of freezing (LeDoux, 2003), to the conditioned stimulus, which can be quantified as a measure of fear learning and memory. Discrete stimuli, such as tones, are often used as conditioned stimuli for fear conditioning; however, olfactory cues are also highly effective at inducing learned freezing (Pavesi et al., 2012; Ross and Fletcher, 2018). This method of associative fear learning differs from those utilizing predator odors, which produces instinctive behaviors rather than learned behaviors, making it ideal for rapidly assessing olfactory learning. Behavioral freezing, defined as absence of all voluntary movements (Blanchard and Blanchard, 1969; Fanselow, 1980), can be measured through automated software that compares pixel differences on a frame-by-frame basis. We developed a protocol that uses the automated FreezeFrame software to deliver discrete olfactory cues during training and testing. This protocol supports standard fear conditioning and subsequent testing but also provides flexibility for expansion to fit broad experimental needs such as extinction, discriminate conditioning, and generalization paradigms or experimental manipulations (e.g., optogenetics or chemogenetics). In addition, olfactory fear conditioning provides a rapid method of studying mechanisms of olfactory associative learning given that training requires few trials in a single day with learning assessed the next day.
Materials and Reagents
- Parafilm
- Tubing
- 1/16” ID Kflex tubing (United States Plastic, catalog number: 65170 )
- 1/16” ID Tygon tubing (Fisher Scientific, catalog number: 14-171-129 )
- 1/8” ID Masterflex Tygon E-Lab (E-3603) Pump Tubing (Cole-Parmer Instrument, catalog number: EW-06509-16 )
- 1/4” ID Tygon tubing (Fisher Scientific, catalog number: 14-171-221 )
- Luers and connectors
- Stopcock 1-way male lock (Cole-Parmer Instrument, catalog number: EW-30600-00 )
- Male Luer x 1/16” hose barb (Cole-Parmer Instrument, catalog number: EW-45518-00 )
- Male Luer Lock Plug (Cole-Parmer Instrument, catalog number: EW-30800-30 )
- Female Luer x 1/8” hose barb (Cole-Parmer Instrument, catalog number: SI-30800-08 )
- Masterflex Y-connector (Cole-Parmer Instrument, catalog number: EW-30614-04 )
- Female Luer 1/16” x 1/16” hose barb (Cole-Parmer Instrument, catalog number: EW-45508-00 )
- (2) 1/4” straight barbed connectors (Cole-Parmer Instrument, catalog number: EW-30612-13 )
- 1/8” NPT male adapter to 1/16” barb (Cole-Parmer Instrument, catalog number: EW-06365-41 )
- 1/8” NPT male adapter to 1/4” barb (Cole-Parmer Instrument, catalog number: EW-30704-09 )
- 1/2” NPT Male adapter to 1/4” Hose Barb (Cole-Parmer Instrument, catalog number: EW-30704-17 )
- Pipette tips (Eppendorf, catalog numbers: 0030073061 , 0030073100 )
- 20 ml glass scintillation vial with polypropylene cap (Sigma-Aldrich, catalog number: Z190535 )
- 16 G 1½ needle (Surgo Surgical Supply, catalog number: 150-305198)
Manufacturer: BD, catalog number: 305198 . - Mice (C57BL/6J; THE JACKSON LABORATORY, catalog number: 000664 ), preferably > 6 weeks old
Notes:- This protocol is suitable for use with other strains, but we recommend users test and determine suitability with their strains.
- If using fear conditioning in conjunction with other techniques, e.g., optogenetics, prepare mice in advance and allow sufficient recovery time prior to training.
- Epoxy (Thorlabs, catalog number: G14250 )
- Mineral oil (Sigma-Aldrich, catalog number: M5904 )
- Odorants, e.g., Ethyl Valerate (Sigma-Aldrich, catalog number: 290866 )
- Alconox (Sigma-Aldrich, catalog number: 242985 )
Equipment
- Single-channel pipettes (Eppendorf, catalog numbers: 3121000074 and 3121000120 )
- Training chamber (Figure 1B)
- Shock floor (Coulbourn Instruments, catalog number: H10-11M-TC-SF )
- Training cage (Coulbourn Instruments, catalog number: H10-11M-TC )
- Metal wall panels (Coulbourn Instruments, catalog number: H90-00M-M-KT01 )
- 25 ft shock cable (Coulbourn Instruments, catalog number: H93-01-25 )
- Precision animal shocker (Coulbourn Instruments, catalog number: H13-15 )
- Testing chamber (custom-made clear Plexiglas chamber, Figure 1C)
- Outer dimensions: 11” x 6” x 5.5” (L x W x H) with 1/2” thick walls.
- A 1/4” thick wall with 2 rows of 6 holes (1/2” dia) beginning ~1/2” from the top to allow air/odor diffusion across the middle wall divides the 11” chamber length in half, such that there are two inner compartments measuring 5” x 5” x 5.5”.
- The chamber has a 1/2” thick lid that can be secured to the chamber by screws.
- The chamber has one 1/2” dia hole on either side of the cage ~1/2” from the top for odor and vacuum lines, respectively.
- USB Cameras (Coulbourn Instruments, catalog number: ACT-VP-02 )
- Actimetrics USB Digital Interface (Coulbourn Instruments, catalog number: ACT-712 )
- FreezeFrame 4 adapter cable for Coulbourn hardware (Actimetrics, catalog number: ACT-INTF )
- Isolation cubicle (Coulbourn Instruments, catalog number: H10-24T )
- Infrared illuminator (Stoelting, catalog number: 60540 )
- Acrylic Flowmeter, 0.1-1 LPM (Cole-Parmer Instrument, catalog number: EW-32460-42 )
- Aquarium air pump, non-UL (Spectrum Brands, Tetra, Whisper®, catalog number: 77851 )
- 2-Channel SPDT Relay Board (Winford Engineering, catalog number: RLY102-12V-DIN )
- 12 V wall power supply for 2-Channel SPDT Relay Board (Winford Engineering, catalog number: WSD050-10-0 )
- Pinch Valves
- 1 Tube Normally Open pinch valve (NResearch, catalog number: 225P021-21 )
- 1 Tube Normally Open pinch valves (NResearch, catalog number: 648P021-82 )
- Olfactory stimulus control (Coulbourn Instruments, catalog number: H15-03 )
- True HEPA Air Purifier, 390 sq. ft. (Honeywell, model: 50250 )
- Cool Moisture Console Humidifier (Honeywell, model: HCM-6009 )
- Humidity and Temperature Monitor (such as FisherbrandTM TraceableTM Thermometer/Clock/Humidity Monitor, Fisher Scientific, catalog number: 06-662-4 )
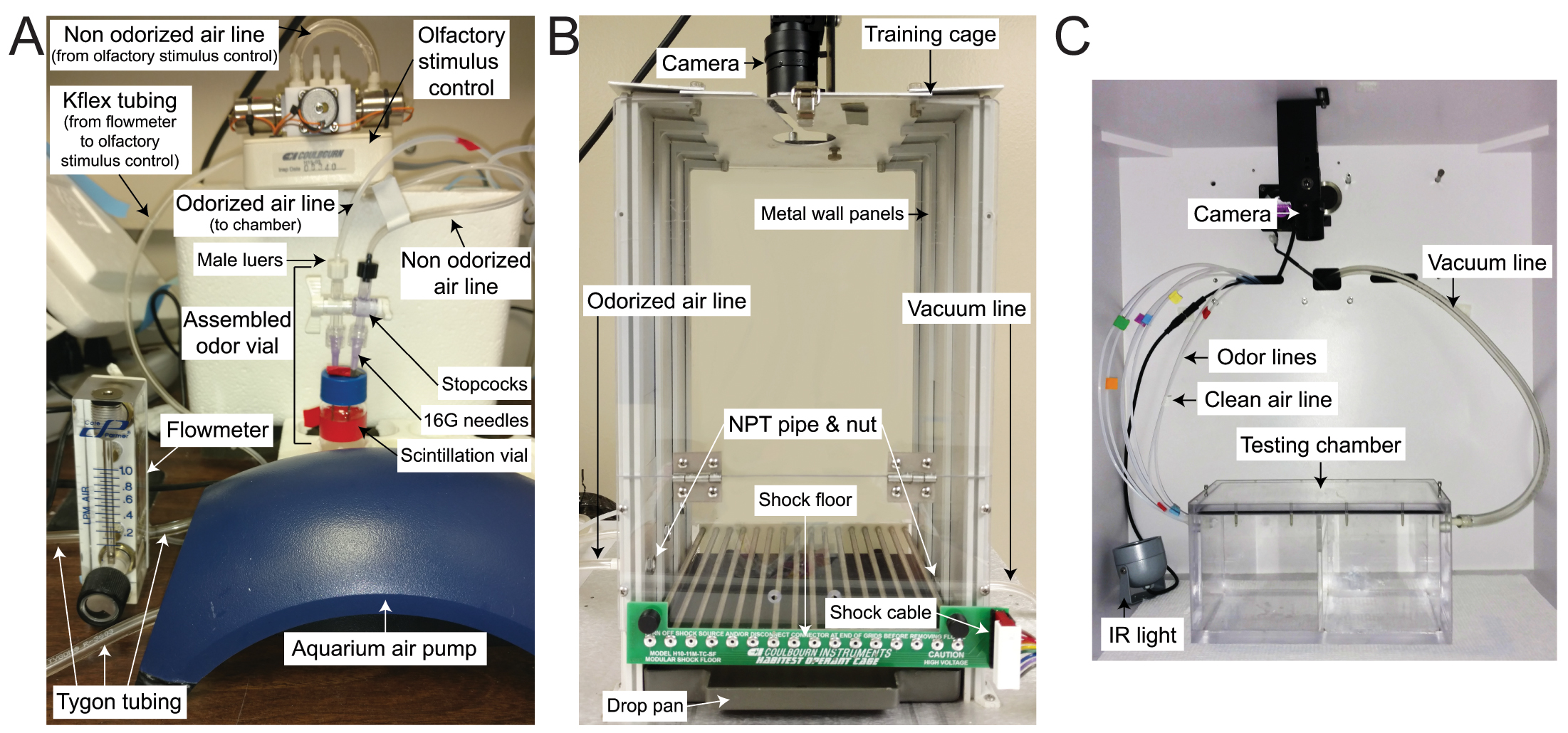
Figure 1. Odor delivery and behavioral chambers. A. The aquarium pump provides air to the flowmeter through Tygon tubing, which is then routed to the olfactory stimulus control for programming delivery. Kflex tubing is attached to the olfactory stimulus control and the non-odorized air stopcock to provide air flow to the assembled odor vial to make odorized air. The odorized air will flow out of the odorized air stopcock and to the attached chamber via additional Kflex tubing. B. The training chamber is modified to add small holes at the left and right of the cage. An NPT pipe is fitted through each hole and secured to the inside of the chamber with a nut. Odorized air lines are attached to the barbed end of the NPT pipe at the left of the cage while a vacuum line is attached to the barbed end of the NPT pipe at the right of the cage, just beneath the shock floor. A camera is positioned above the cage for recording. C. The testing chamber is placed inside the isolation cubicle with an infrared light source positioned on the right of the chamber and a camera mounted above the chamber. Separate tubing carrying clean and odorized air enter the chamber on the left and a vacuum is attached to the right of the chamber to facilitate odor clearance.
Software
- FreezeFrame 4 Software (Coulbourn Instruments, catalog number: ACT-100A)
Procedure
Notes:
- Assemble behavioral training chamber according to the manufacturer’s instructions and optimize for your needs.
- Set up cameras and program the delivery of stimuli [odor(s), shock(s), air, vacuum, etc.] and duration of training and testing protocols into FreezeFrame by following the instructions provided with the software.
- This protocol will detail training and testing murine olfactory fear learning with a single odor, ethyl valerate (E5), but can be adapted to test multiple odors by adding additional odor vials and lines to the testing chamber (see Ross and Fletcher, 2018). It could be expanded for use with discriminate conditioning by adding additional odor vials and lines to the training chamber. Users can also add consecutive testing days to assess extinction if desired.
- Modify the training and testing chambers
- Use a drill to make a hole into one side of the training chamber for the vacuum line. The hole should be situated at the middle of the chamber between the shock floor and drop pan. Insert the 1/2” NPT male pipe adapter with 1/4” barb, which should fit snugly, into the drilled hole from the outside of the chamber and secure with a 1/2” NPT nut. The nut should be on the inside of the chamber and the barb should extend from the outside of the chamber (Figure 1B).
- Use a drill to make a second smaller hole in the opposite side of the training chamber for the odor line. The hole should also be situated at the middle of the chamber approximately 1/2-1” above the shock floor. Insert the 1/8” NPT male pipe adapter with 1/16” barb, which should fit snugly, into the drilled hole from the outside of the chamber and secure with a 1/8” NPT nut. The nut should be on the inside of the chamber, and the barb should extend from the outside of the chamber (Figure 1B).
- Place the testing chamber inside the isolation cubicle and run all tubing/cords through the holes on the back of the chamber to the inside (Figure 1C).
- Place the infrared light source inside the cubicle towards the side of the testing chamber that will not hold the mouse/the side that odorized air will enter the chamber. Situate to minimize glare on the chamber/camera by pointing the light source directly at a wall of the isolation cube rather than the camera or testing chamber (Figure 1C).
- Insert and thread the NPT end of the 1/2” NPT Male Adapter to 1/4” Hose Barb into one of the holes on the sides of the testing chamber. Attach the vacuum line to the barbed end (Figure 1).
- Assemble odor vial(s)
- Remove 16 G 1½ needles from individual packaging and lock a stopcock onto the female Luer side of each needle.
- If not already separated, remove the plastic cap from the 20 ml scintillation vial. Drill two holes into the top of the cap, leaving enough space between the holes for the needles with attached stopcocks. The holes should be as close to the size of the needles as possible. Secure the cap with drilled holes onto the bottle.
- Carefully uncap the needles and insert the beveled end through the holes on the cap, so that the needles are inside the bottle. Needles should fit snugly in drilled holes.
- Mix epoxy and apply to the cap of the bottle liberally to secure the needles to the cap. Make sure not to get epoxy on the stopcocks or the bottle itself. Let cure overnight.
- Use a sharpie to mark one of the stopcocks. This stopcock will act as the “odorized air” side of the odor vial. The other stopcock will receive non-odorized air from the Olfactory Stimulus Control.
- Cut a length of Kflex tubing that will fit your specific setup (long enough to stretch from bottles to chambers without excessive tension). In one end of the Kflex tubing, insert the 1/16” hose barb end of a male Luer. Attach the male end of the Luer to the top of the marked stopcock and attach the other end of the Kflex tubing to the training or testing chamber as desired.
- It can be difficult to detach tubing from barbed luers; therefore, is advantageous to have a separate length of tubing from the odor vial(s) to each chamber.
- If using more than one odor for testing, Kflex tubing should be similar length for all odor vials to testing chamber. Multiple tubes can be secured to one another with parafilm and inserted into the hole on the side of the testing chamber.
- Assemble clean air and odorized air delivery system
- Attach one end of the Masterflex Tygon tubing to one of the aquarium air pump outputs and the other end of the Tygon tubing to the input of the flowmeter. On a second piece of Tygon tubing, connect the 1/8” female Luer and cap it off with the corresponding male Luer lock plug. Attach the other end to the opposing flowmeter input.
- Attach one end of a short piece of the 1/16” Tygon tubing to the flowmeter output, in the other, place the Masterflex Y-connector. Cut two more pieces of the 1/16” Tygon tubing, approximately equal lengths, and join to the remaining barbs on the Y-connector. One of these lines will supply the air for the “clean air” line while the other will supply air for the “odor” line(s).
- The tubing designated for “clean air” should be paired with a Normally Open pinch valve with 1/16” ID tubing, while the tubing for the “odorized air” line should be paired with the Olfactory Stimulus Control. Fit the end of the “clean air” line with Luers to connect to the pinch valve tubing. Use a combination of 1/16” male and female Luers to adjoin (optionally, you can use straight barbed connectors with 1/16” barbs on both sides).
- After attaching the “odorized air” tubing to the back of the Olfactory Stimulus Control, connect another length of tubing to one of the three ports on top of the Olfactory Stimulus Control. Place a 1/16” male Luer to the other end of the tubing so that it can be connected directly to the unmarked stopcock on the odor vial(s).
- Wire the pinch valve to 2-Channel SPDT Relay Board, making sure to match the pinch valve type (NO or NC for Normally Open and Normally Closed, respectively) to the correct contact.
Notes:- For more information regarding wiring of relay boards, please refer to the schematics provided on the Winford website.
- In order to minimize noise emitted by the pinch valve during training and testing, we place the pinch valve inside a styrofoam or plastic (such as a used pipette tip) box with foam or styrofoam peanuts. We drill small holes in the side of the box for the pinch valve wires and mount the relay board to the top (lid) of the box.
- Using a combination of 1/16’ male and female Luers (or 1/16” straight barbed connectors), connect the remaining “clean air” pinch valve tubing to 1/16” Kflex tubing. Direct and attach this tubing to the training or testing chamber as needed.
Note: Again, it may be advantageous to have separate “clean air” lines for the training and testing chambers that can be attached to the pinch valve separately.
- Assemble vacuum system
- Wire the 1/4” ID tubing pinch valve to a 2-Channel SPDT Relay Board (as before) and enclose in a container for noise reduction if desired.
- Fit one end of 1/4” ID Tygon tubing directly onto laboratory vacuum nozzle. Place a 1/4” straight barbed connector into the other end and attach directly to the pinch valve tubing.
- Place the second 1/4” straight barbed connector into the opposite end of the pinch valve tubing and connect it to another length of 1/4” ID Tygon tubing.
- Attach this end of the vacuum line to the training or testing chamber as needed.
Note: It can be difficult to detach tubing from barbed connectors. Optionally, you can cut two lengths of 1/4” ID Tygon tubing and designate one for the training chamber and one for testing chamber. In this case, use a combination of 1/4” male and female Luers to join the pinch valve and vacuum tubing in Step D3. Attach each length of vacuum tubing to the appropriate chamber and connect to the pinch valve as needed.
- Program FreezeFrame protocols for stimulus delivery
- Open the FreezeFrame Recorder and create a training protocol
Note: Refer to the FreezeFrame software manual provided by Coulbourn for additional information regarding FreezeFrame setup. - The training protocol should consist of 6 odor-shock pairings that occur every other minute starting in the second minute of the training protocol (Figure 2A). The odor presentations should last 10 sec each with the shock occurring 0.5 sec before the end of the odor presentation and lasting until the end of the odor presentation (i.e., Odor 60-70 sec, shock 69.5-70 sec; Odor 180-190 sec, shock 189.5-190 sec, etc.). Clean air and vacuum should be on at all times except during odor presentations (i.e., Clean air/vacuum 0-60 sec; 70-179 sec, etc.). Approximate training time = 720 sec.
- To test learned fear for just the training odor, the protocol should consist of 2 presentations of the conditioned odor (Figure 2B). Presentations should last for 20 sec every 240 sec starting in the second minute of the testing protocol (i.e., #1 60-80 sec; #2 300-320 sec). Clean air and vacuum should be on at all times except during odor presentations (i.e., Clean air/vacuum should be on from 0 sec to 59 sec and 120 sec to 299 sec but programmed to be off from 60 to 119 and 300 to 359)
Note:- The testing protocol can be adapted to increase the number of presentations and/or to test multiple odors. Adding epochs 240 sec apart with 20 sec presentations as needed.
- Remember, the clean air and vacuum lines are attached to NO pinch valves, meaning the trigger from the computer will signal to close the pinch valve, thus shutting the air and vacuum off.
- Open the FreezeFrame Recorder and create a training protocol
- Prepare odor(s) for training/testing
Note: Prepare odor(s) fresh daily.- Empty any waste odorant into an appropriate container.
- Inside a fume hood, dilute E5 in mineral oil to ~200 ppm (64 μl E5 in 1,936 μl mineral oil) inside the odor vial. Gently shake to mix.
Note: Any additional odorants should also be intensity matched to ~200 ppm, which will require different ratios of odor: mineral oil, to be determined by users. - Place the non-odorized air line (from the Olfactory Stimulus Control) on the unmarked stopcock and the “odorized air” Kflex tubing on the marked stopcock. Make sure to open stopcocks during training and testing when odor should reach chambers.
- Olfactory fear conditioning (training)
- Turn on humidifier and HEPA filter. If humidity is low, allow enough time before training to ensure humidity is > 35%. Leave both running throughout training.
- Ensure that the appropriate camera is plugged into the computer and open the FreezeFrame Recorder.
- Turn on vacuum, aquarium pump, pinch valves, and Precision Animal Shocker and make sure all necessary tubing is attached to the training chamber. Prepare fresh odor (above), attach necessary tubing, and open the stopcocks on the odor vial.
- Set the Shocker to deliver 0.8 mA shocks with 8 pole scanned output. Make sure that the shocker switch is positioned to “Remote” (under “Operate”) and “Subject.”
Note: Other shock intensities may be used, to be determined by the user. We have elicited fear learning using 0.4 mA and 0.6 mA in addition to 0.8 mA. - Use the Stimulus Configuration Panel under the Settings tab to ensure all stimuli are functioning and will be triggered appropriately during training.
- Select the training protocol from the dropdown menu.
- Specify a data file for storing data.
- Enter a unique name for each mouse (ideally this should relate to a physical identifier such as tail mark, ear punch, etc.) and take the reference frame.
Note: If transporting mice from animal facility, allow at least 30 min before subjecting to training. - Place mouse in the chamber and allow 5 min for habituation.
- Press start and observe mice during training for signs of shock experience (jumping, squeaking, increased freezing, etc.).
- Remove mouse after completion of training protocol and return to the cage.
Note: We wait approximately 24 h after training before testing mice; however, different time points could be useful for determining short-term vs. long-term memory or retention. - Clean training chamber with 1% Alconox solution and water, making sure to wipe down the shock floor and drop pan before starting next mouse.
- Olfactory fear testing
- Ensure that the appropriate camera is plugged into the computer and open the FreezeFrame Recorder.
- Turn on vacuum, aquarium pump, pinch valves, and infrared light and make sure all necessary tubing is attached to the testing chamber. Prepare fresh odor (above), attach necessary tubing, and open the stopcocks on the odor vial.
Note: If using multiple odors during testing, move the non-odorized air line to each odor vial shortly before presenting that odor and open stopcocks. The non-odorized air line provides air to the odor vial that will become your odorized air for odor presentations. Make sure all stopcocks on other odor vials are closed when not being presented. - Use the Stimulus Configuration Panel under the Settings tab to ensure all stimuli are functioning and will be triggered appropriately during testing.
- Select the testing protocol from the dropdown menu.
- Specify a data file for storing data.
- Enter the same unique name for each mouse as the previous day (to allow matching between training and testing data if desired) and take the reference frame.
Note: If transporting mice from animal facility, allow at least 30 min before subjecting to training. - Place mouse in the chamber and allow 5-10 min for habituation. During this time, observe mice for behaviors indicative of comfort and exploration, such as rearing and grooming. Mice that exhibit excessing freezing during the first minute of testing will be removed from the analysis (see below), so it is important to provide sufficient time during habituation for any novel context-induced freezing to subside.
- Press start and allow the mouse to complete testing protocol.
- Remove mouse after completion of training protocol and return to the cage.
- Clean testing chamber with 1% Alconox solution and water before starting next mouse.
Data analysis
- Open the FreezeFrame Trial Viewer and locate the appropriate testing data file.
- Select the desired mouse from the Animal box to the left of the screen (Video 1).Video 1. Example of testing data from FreezeFrame4 Trial Viewer. This video depicts 120 sec of olfactory fear testing 24 h after olfactory fear conditioning with all of the data provided from FreezeFrame4 (video playback in real time). The conditioned odor is presented at 60 sec for 20 sec and the clean air and vacuum are turned off at 60 sec for a total of 60 sec. The Animal menu on the left shows all Animal IDs recorded under the same data file, with the selected trial highlighted in blue. The Motion Index graph indicates the recorded activity (y-axis; yellow line) across time (x-axis). The vertical blue line moves in real time with the video while the horizontal blue line shows the user-defined threshold for freezing. The rectangle below shows bouts of freezing, as determined by user-defined threshold and bout, as yellow boxes. Periods of non-freezing are not colored. The next rectangle below provides a visual for the protocol and shows the onset/offset of all stimuli during the protocol (blue rectangle: odor onset, yellow rectangle: vacuum offset, green rectangles: clean air offset). The motion index histogram should be used to set threshold. The bout length can be set to fit specific needs; in this case, 2 sec. The video to the right of the histogram shows the behavior of the mouse throughout testing, with bouts of freezing (determined through the user-defined threshold and bout length) indicated by a green light above. In this example, the mouse freezes for 0% of the 1st 60 sec interval (baseline freezing) and 74.28% during the 2nd 60 sec interval during the presentation of the conditioned stimulus. (All experimental protocols were approved by the University of Tennessee Institutional Animal Care and Use Committee.)
- Look at the Motion Index graph in the lower left corner. Use your cursor to drag the blue vertical line inside the graph to the first trough of the yellow line. This will set your freezing threshold.
Note: In our hands when sampling at 7.5 Hz, thresholds typically occur at motion index values between 2 and 5, and no higher than 10 (Video 1). - Manually set the bout to 2 sec (Video 1).
Note: This indicates the motion index must be below the set threshold for a total of 2 sec to be counted as a bout of freezing. - Navigate to the Analysis tab and record the % Freezing in 60 sec intervals for the duration of the testing protocol for each mouse.
Note: Optionally, you may set the same threshold for all mice and export all the analysis for all mice into a single spreadsheet. - Exclude any mice that freeze excessively in the first minute of the testing protocol from analysis. Additionally, mice should freeze during odor cue but should decrease freezing behaviors as the odor is evacuated from the chamber before the next cue (Figure 2C).
Note: Based on data collected in our lab, we exclude any mice that freeze more than 20% in the first minute, but this can be adjusted based on specific needs of individual users. High freezing during the first minute indicates high generalized anxiety and can make it difficult to determine whether freezing during odor presentations is specific to the odor cue. - For each mouse, average together the % freezing values during presentations of E5 (i.e., if you presented E5 twice during testing from 60 sec to 120 sec and again from 300 sec to 360 sec, average the two corresponding values together to get an average % freezing for each mouse).
Note: If you presented two separate odors twice each during testing, average the two values for the 1st odor together and the two values for the 2nd odor together separately so you have an average % freezing for each mouse for each odor; if you presented multiple odors only once each, do not average. - Average the E5 % freezing values for all mice together to generate a group average.
- Average the % freezing during the first minute of testing for all mice together to generate a group average of baseline freezing.
- Use a paired samples t-test to compare the E5-induced freezing to the baseline freezing. Significant results demonstrate learned fear as a result of fear conditioning to E5 (Figure 2D).
Note: If you are interested in comparing acquired freezing between different experimental groups, you may use appropriate tests to statistically compare E5-induced freezing between groups. Additionally, if you test freezing to multiple odors, you may use appropriate tests to compare freezing to one odor with that to others.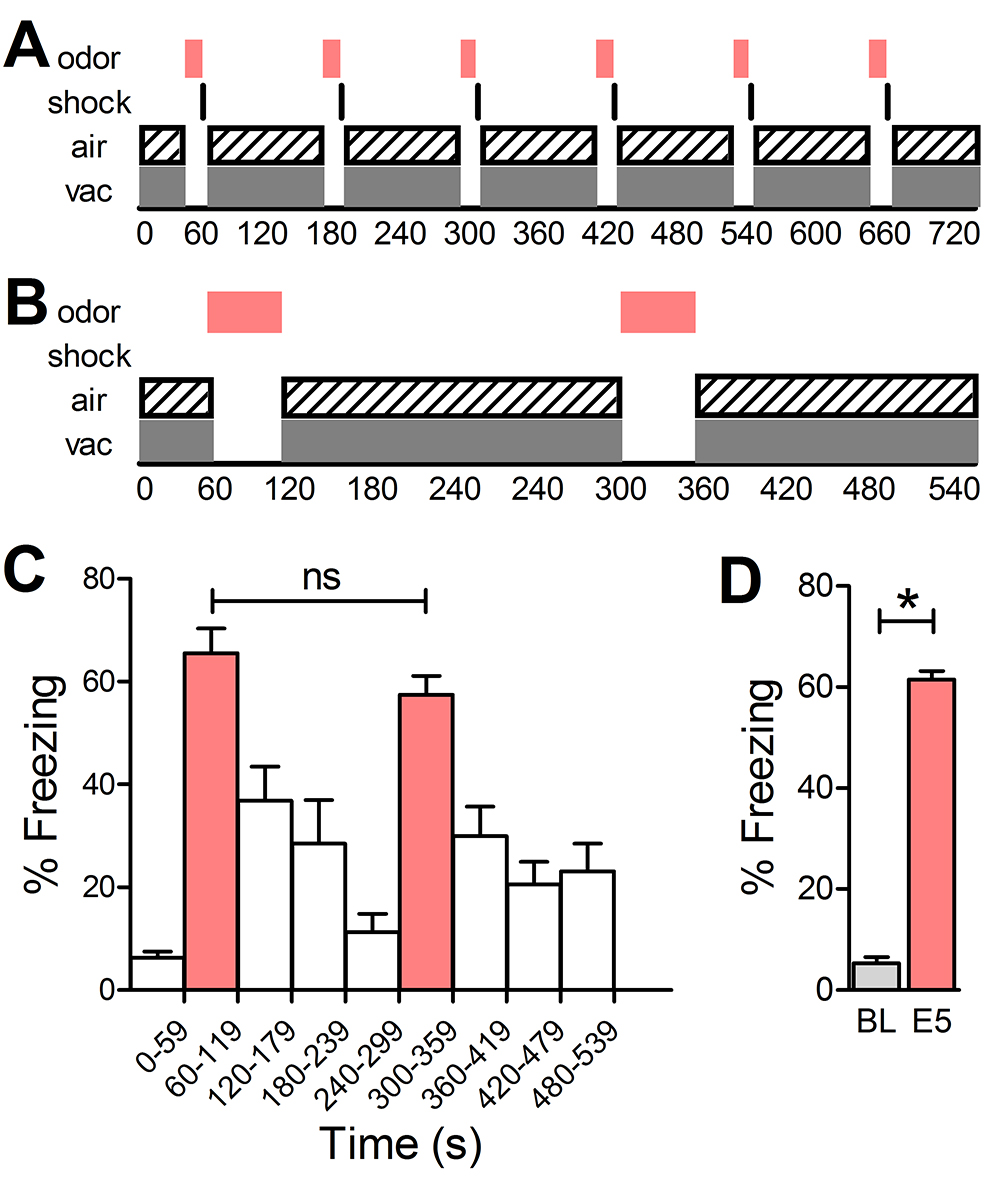
Figure 2. Training and testing schematics and representative testing analysis. A. Schematic for training paradigm illustrating timing of each stimulus. B. Schematic for testing paradigm illustrating timing of each stimulus. C. Representative testing data demonstrating % freezing behaviors of B6 mice (n = 10) during 60 sec bins throughout testing paradigm. Colored bars represent 60 sec bins during which E5 was filling chamber. Uncolored bars represent 60 sec bins during which air and vacuum are on and odor is not filling chamber. Freezing does not differ significantly between the first and second presentations of E5. D. Representative testing data demonstrating average freezing behaviors during baseline 1st minute and for both 60 sec E5 presentations (n = 10). Mice freeze significantly more during presentations of E5 than during the baseline minute, indicating acquired fear to the conditioned stimulus. Error bars denote SEM, *P < 0.05.
Notes
- Rarely, control mice will unexpectedly exhibit very low freezing to the conditioned stimulus; therefore, we do not include control mice in analysis that do not freeze at least 25% to the first presentation of the conditioned stimulus. These mice are deemed non-learners.
- Aged mice typically freeze less but we observe consistent freezing of at least 50% to the conditioned stimulus up to 8 months of age in control mice.
Acknowledgments
This publication was supported by the NIDCD awards R01DC013779 to MLF and F31DC016485 to JMR. An adapted form of this protocol for testing fear generalization was used in Ross and Fletcher (2018), which utilized multiple intensity matched odors (~200 ppm as measured by a photoionization detector, Rae Systems, catalogue number: 059-4020-000) during testing (Sigma-Aldrich, catalogue numbers: E15701, 148962, 418099, 537683, 00790).
Competing interests
The authors declare that they have no conflict of interest.
Ethics
All experimental protocols were approved by the University of Tennessee Institutional Animal Care and Use Committee.
References
- Blanchard, R. J. and Blanchard, D. C. (1969). Crouching as an index of fear. J Comp Physiol Psychol 67(3): 370-375.
- Fanselow, M. S. (1980). Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15(4): 177-182.
- LeDoux, J. (2003). The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23(4-5): 727-738.
- Pavesi, E., Gooch, A., Lee, E. and Fletcher, M. L. (2012). Cholinergic modulation during acquisition of olfactory fear conditioning alters learning and stimulus generalization in mice. Learn Mem 20(1): 6-10.
- Ross, J. M. and Fletcher, M. L. (2018). Learning-dependent and -independent enhancement of mitral/tufted cell glomerular odor responses following olfactory fear conditioning in awake mice. J Neurosci 38(20): 4623-4640.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ross, J. M. and Fletcher, M. L. (2018). Assessing Classical Olfactory Fear Conditioning by Behavioral Freezing in Mice. Bio-protocol 8(18): e3013. DOI: 10.21769/BioProtoc.3013.
- Ross, J. M. and Fletcher, M. L. (2018). Learning-dependent and -independent enhancement of mitral/tufted cell glomerular odor responses following olfactory fear conditioning in awake mice. J Neurosci 38(20): 4623-4640.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Neuroscience > Behavioral neuroscience > Olfaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link