- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Behavioral Assay to Examine the Effects of Kavalactones on Caenorhabditis elegans Neuromuscular Excitability
(*contributed equally to this work) Published: Vol 8, Iss 18, Sep 20, 2018 DOI: 10.21769/BioProtoc.3008 Views: 5230
Reviewed by: Pengpeng LiNarayan SubramanianManish Chamoli

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Artificial Optogenetic TRN Stimulation of C. elegans
Ithai Rabinowitch [...] Jihong Bai
Oct 20, 2016 8620 Views

Pentylenetetrazole (PTZ)-induced Convulsion Assay to Determine GABAergic Defects in Caenorhabditis elegans
Shruti Thapliyal and Kavita Babu
Sep 5, 2018 6152 Views

Aerotaxis Assay in Caenorhabditis elegans to Study Behavioral Plasticity
Qiaochu Li [...] Karl Emanuel Busch
Aug 20, 2022 2251 Views
Abstract
Kavalactones are a class of lactone compounds found in Kava, a traditional beverage from the South Pacific Islands that is derived from the root of Piper methysticum. When consumed, these compounds produce sedative and anxiolytic effects, suggesting their potent actions on the nervous system. Here, we provide a protocol to examine the effects of kavalactones on C. elegans neuromuscular excitability. Our methodology could provide insight into the neurophysiological actions of kavalactones.
Keywords: KavaBackground
Kava, a tranquilizing beverage from the Pacific Islands, has been consumed by Pacific Islanders for centuries (Rowe et al., 2011; Kautu et al., 2017). Kava contains a group of lipophilic compounds called kavalactones, which are believed to be responsible for the sedative, anxiolytic, and other therapeutic effects of the drink (Rowe et al., 2011; Savage et al., 2015; Kautu et al., 2017). Here, we provide a protocol to examine the effects of kavalactones on neuromuscular activity, using C. elegans as a model system (Kautu et al., 2017). In our assay, we showed that administration of aqueous kavalactone solution induced epileptic-like convulsions and paralysis in a dose-responsive manner. These manifestations are suggestive of the modulatory actions of kavalactones on the neuromuscular junction. Thus, our protocol could provide important insight into the neurophysiological actions of kavalactones.
Materials and Reagents
- Petri dishes, sterile (Carolina Biological Supply, catalog number: 741248 )
- 2 ml centrifuge tubes (Thomas Scientific, catalog number: 111572LK )
- P1000 pipette tips, sterile (Carolina Biological Supply, catalog number: 215060 )
- P200 pipette tips, sterile (Carolina Biological Supply, catalog number: 215050 )
- 0.5 L bottle
- E. coli OP50 (Carolina Biological Supply, catalog number: 155073 )
- C. elegans N2 (wild-type)
- Kava pills (Gaia Herbs, catalog number: 90A10060 )
- NaCl (Sigma-Aldrich, CAS: 7647-14-15)
- Peptone (Sigma-Aldrich, catalog number: 70176-100G )
- Difco Bacto agar (Carolina Biological Supply, catalog number: 156783B )
- CaCl2 dihydrate (Fisher Scientific, catalog number: C79 500 )
- MgSO4 (Sigma-Aldrich, CAS: 7487-88-9)
- Potassium Phosphate Monobasic (KH2PO4) (Sigma-Aldrich, CAS: 7778-77-0)
- Potassium Phosphate Dibasic (K2HPO4) (Sigma-Aldrich, CAS: 7758-11-4)
- Cholesterol (Fisher Science Education, CAS: 57-88-5)
- Hypochlorite solution (Sigma-Aldrich, CAS: 7681-52-9)
- NaOH (Sigma-Aldrich, CAS: 1310-73-2)
- 95% ethanol (Sigma-Aldrich, CAS: 64-47-5)
- 1 M CaCl2 stock solution (see Recipes)
- 1 M MgSO4 stock solution (see Recipes)
- 1 M Potassium Phosphate stock solution (pH 6) (see Recipes)
- 5 mg/ml Cholesterol stock (see Recipes)
- Nematode Growth Medium (NGM) Agar plates (see Recipes)
- Bleach sodium hypochlorite solution (see Recipes)
- Kava stock solution (5 mg/ml) (see Recipes)
Equipment
- P200 and P1000 micropipette
- Sharp Edged Scissors
- Autoclave
- Bench-top Micro Centrifuge (Oxford Lab Products, model: C12V )
- Meiji EMT Stereomicroscope on PBH Stand (Meiji Techno, models: EMT-1 , PBH Stan d, MA502 )
- Microscope Digital Camera (OMAX Microscope, catalog number: A3550UPA-R75 )
- Platinum wire worm pick (Genesee Scientific, catalog number: 59-AWP )
- Sterile incubator
Software
- OMAX ToupView 3.7
- Microsoft Office 2010 Excel (Microsoft Corporation, Redmond, USA)
Procedure
- Synchronization of C. elegans via sodium hypochlorite treatment
- First, allow gravid adult N2 (wild-type) worms to grow at 20 °C and lay embryos on NGM plates seeded with E. coli OP50 strain (Brenner, 1974). Once there is a sufficient number of embryos to be used for the assays, the worm stages should be collected, washed, and synchronized. To collect worms, use a P1000 micropipette to dispense 1,000 μl of DI water onto the plate to pool the worms and embryos into one spot. Pipette the water containing the worms into a 2 ml microcentrifuge tube and centrifuge at room temperature for 1 min at 7,558 x g. Carefully pipette off the excess liquid without disturbing the worm pellet.
Note: To avoid strain loss, a few worms should be transferred to a seeded NGM plate prior to collection and synchronization; all worms from this point on will be used in the assay. - Prior to synchronizing the worms, dilute 20% stock of bleach sodium hypochlorite solution to 10-15% using distilled water. Synchronize the worms by incubating them in the diluted bleach sodium hypochlorite solution (10-15%) for 3-5 min.
- Centrifuge the tube at room temperature for 30 sec at 7,558 x g. Carefully remove excess bleach sodium hypochlorite solution by pipetting. Resuspend the pellet using 500 μl of DI water. Centrifuge at room temperature for another minute at 7,558 x g. Remove excess DI water, leaving the pellet intact.
Note: Do not incubate the worms for more than 5 min, including time in the centrifuge. Also, we found that carryover bacteria does not interfere with the assay. However, an excessive amount of carryover bacteria must be avoided. An experimenter can do this by controlling the amount of bacteria that is initially seeded on the plate. In general, we seed 100 x 200 mm NGM plates with 150-200 μl of OP50 bacteria. - Collect the embryos and the dead worms from the microcentrifuge tube using a P200 or P1000 micropipette and transfer them to a new NGM plate seeded with OP50 bacteria. It is best to transfer the dead worms and the embryos to the edge of the seeded plate not covered with OP50 bacteria.
- Allow the embryos to hatch and grow until the L4 to young adult, stage. This will take approximately two days.
- First, allow gravid adult N2 (wild-type) worms to grow at 20 °C and lay embryos on NGM plates seeded with E. coli OP50 strain (Brenner, 1974). Once there is a sufficient number of embryos to be used for the assays, the worm stages should be collected, washed, and synchronized. To collect worms, use a P1000 micropipette to dispense 1,000 μl of DI water onto the plate to pool the worms and embryos into one spot. Pipette the water containing the worms into a 2 ml microcentrifuge tube and centrifuge at room temperature for 1 min at 7,558 x g. Carefully pipette off the excess liquid without disturbing the worm pellet.
- Treatment of worms with aqueous kavalactone solutions
- Transfer L4 to young adult worms to 2 ml microcentrifuge tubes. This can be done using the same method of washing and collecting described previously.
- Prepare 0 mg/ml, 0.2 mg/ml, 0.4 mg/ml, 0.6 mg/ml, 0.8 mg/ml and 1.0 mg/ml kavalactone solutions by diluting the stock solution (5 mg/ml) with distilled water.
- Suspend worms in 500 μl of the prepared kavalactone concentrations (above).
Note: Our kavalactone solution was made using a kavalactone supplement purchased from Gaia Herbs, Inc. (Brevard, NC, USA). Stocks of kavalactone solutions can be stored for up to 2 months at 4 °C. It is important to shake and mix the kavalactone solution well before using it. - Incubate the worms in the pre-warmed kavalactone solution (in the 2 ml microcentrifuge tubes) for 30 min at room temperature. During incubation gently invert the tubes 5-8 times. Do this 5 times during the 30-min time span.
- Following the 30-min incubation, centrifuge the tubes at room temperature for 2 min at approximately 7,558 x g.
- Remove excess solution, while leaving the pellet of worms intact.
- Rinse the worms with 200 μl of distilled water, resuspending the pellet, and centrifuge again at room temperature for an additional 2 min at 7,558 x g.
- Remove the excess liquid, leaving a sufficient amount to transfer the worms. Using a P200 or P1000 micropipette, transfer approximately 40 worms from the kavalactone solution to new, dry 60 mm NGM plates without E. coli bacteria.
Note: Worms can be lost during the resuspension/transfer process, so it’s a good idea to start with a higher number of worms than necessary for the assay to account for loss.
- Scoring worms for convulsions and paralysis
- Use a dissecting/stereo microscope to count the worms as they are being transferred to the clean 60 mm NGM plate.
- Allow the worms to acclimate to the new environment for 15 min before scoring. Here, the experimenter can spread out the worms using a worm pick to prevent them from clumping together in one spot.
- When scoring, observe the worms for convulsions and paralysis. The convulsions manifest as full body repetitive muscle contractions and paralysis. Paralysis is characterized as no visible movement upon observation. Since many convulsing worms progressively become paralyzed over the course of time, we treated convulsions and paralysis as one variable in this particular assay (see Figure 1).
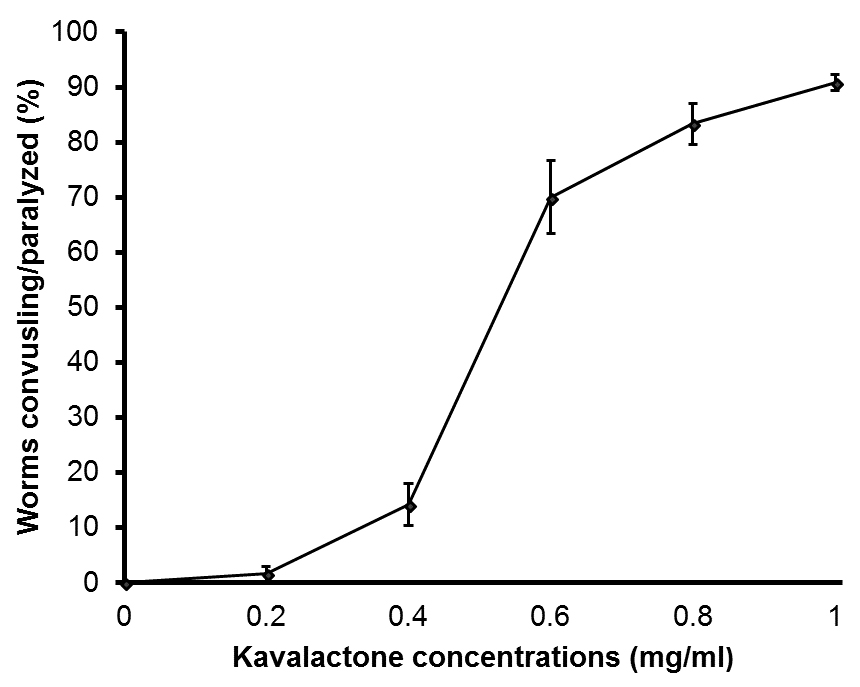
Figure 1. A dose-response curve of kavalactone-induced convulsions and paralysis in C. elegans N2 (wild-type) worms. Three independent experiments were performed at each concentration and the response levels of the worms were averaged and converted to percent. For each experiment (at each concentration) 40 worms were scored (n = 120). At 0 mg/ml, C. elegans worms were dissolved in DI water without kavalactones (negative control). All error bars indicate standard deviations (SD) for the 3 averaged experiments. - Record movies of convulsing and paralyzed worms after kavalactone treatment for a minimum of 30 sec, using a suitable video camera or device. Next, observe and quantify the number (percentage) of worms exhibiting any of the following responses: anterior-posterior full body convulsions (Video1), progression from anterior-posterior full body convulsions to full body paralysis (Video 2), and full body paralysis (Video 3):Video 1. A wild-type N2 worm exhibiting strong full body convulsions (repetitive anterior-posterior muscle contractions) when treated with 1 mg/ml kavalactone aqueous solutionVideo 2. A wild-type N2 worm showing progression from convulsion to full body paralysis when exposed to 1 mg/ml kavalactone aqueous solutionVideo 3. A wild-type N2 worm showing full body paralysis when treated with 1 mg/ml kavalactone aqueous solution
Data analysis
We calculated percent averages and standard deviations for all experiments using Microsoft Office 2010 Excel (Microsoft Corporation, Redmond, USA).
Notes
This protocol can be adapted for other C. elegans mutants of interest.
Recipes
- 1 M CaCl2 stock solution
Dissolve 7.35 g of CaCl2 in 50 ml of DI H2O
Autoclave for 30 min at 121 °C
Store at room temperature - 1 M MgSO4 stock solution
Dissolve 6.02 g of MgSO4 in 50 ml of DI H2O
Autoclave for 30 min at 121 °C
Store at room temperature - 1 M Potassium Phosphate (pH 6) stock solution
Dissolve 35.6 g of K2HPO4 and 108.3 g of KH2PO4 in 1 L of DI water
Autoclave for 30 min at 121 °C
Store at room temperature - 5 mg/ml Cholesterol stock
Add 250 mg of cholesterol to 50 ml 95% ethanol
Mix on a rotator until fully dissolved
Store the cholesterol solution at room temperature - Nematode Growth Medium (NGM) Agar plates-recipes
Add 1.2 g of NaCl, 1.0 g of peptone, and 6.8 g of Difco Bacto agar to 400 ml of DI H2O in a 0.5-L bottle
Autoclave for 25 min at 121 °C
Allow media to cool to approximately 55-60 °C, then add 400 μl of 1 M CaCl2, 400 μl of 1 M MgSO4, 10 ml of potassium phosphate (pH 6), and 400 μl of 5 mg/ml cholesterol
Mix gently after adding each component
Store plates in a sterile 20 °C incubator - Bleach sodium hypochlorite solution
Combine 8.25 ml of DI H2O, 3.75 ml of 1 M NaOH, and 3.0 ml of sodium hypochlorite
Store at room temperature - Kava stock solution (5 mg/ml)
Dissolve 1 kava pill in 15 ml of DI H2O
Store at 4 °C
The pill (caplet) can be cut open with scissors
Acknowledgments
This study was supported by the Biology Department and the Catalyst Fund of the Math and Science Division of Greenville University. This protocol was adapted and modified from a previous study published in 2017 (Kautu et al., 2017).
Competing interests
The authors declared no competing or conflicts of interests.
References
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77(1): 71-94.
- Kautu, B. B., Phillips, J., Steele, K., Mengarelli, M. S. and Nord, E. A. (2017). A behavioral survey of the effects of kavalactones on Caenorhabditis elegans neuromuscular transmission. J Exp Neurosci 11: 1179069517705384.
- Rowe, A., Zhang, L. Y. and Ramzan, I. (2011). Toxicokinetics of kava. Adv Pharmacol Sci 2011: 326724.
- Savage, K. M., Stough, C. K., Byrne, G. J., Scholey, A., Bousman, C., Murphy, J., Macdonald, P., Suo, C., Hughes, M., Thomas, S., Teschke, R., Xing, C. and Sarris, J. (2015). Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials 16: 493.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kautu, B. B., Chappel, J., Steele, K., Phillips, J. and Mengarelli, M. S. (2018). A Behavioral Assay to Examine the Effects of Kavalactones on Caenorhabditis elegans Neuromuscular Excitability. Bio-protocol 8(18): e3008. DOI: 10.21769/BioProtoc.3008.
Category
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









