- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
An Inexpensive and Comprehensive Method to Examine and Quantify Field Insect Community Influenced by Host Plant Olfactory Cues
Published: Vol 8, Iss 16, Aug 20, 2018 DOI: 10.21769/BioProtoc.2967 Views: 6456
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Quantification of Mandelonitrile from Arabidopsis thaliana Using Gas Chromatography/Mass Spectrometry
Ana Arnaiz [...] Isabel Diaz
Jun 20, 2023 1565 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1171 Views

A Step-by-Step Computational Protocol for Functional Annotation and Structural Modelling of Insect Chemosensory Proteins
Rajeswari Kalepu [...] Nor Azlan Nor Muhammad
Nov 20, 2025 1664 Views
Abstract
Insect pollinators, herbivores and their natural enemies use olfactory cues emitted by their host plants to locate them. In insect-plant ecology, understanding the mechanisms underlying these interactions are of critical importance, as this bio-communication has both ecological and agricultural applications. However, the first step in such research is to identify and quantify the insect community associated with the plant/s species of interest. Traditionally, this has been accomplished by a variety of insect trapping methods, either using pitfall traps, or sticky traps, or sweep nets in field. The data collected from these traps tend to be incomplete, and also damage the specimens, making them unusable for any taxonomic purposes. This protocol derives ideas from these traditional traps and use a combination of three easily made inexpensive modified traps that conceals the host plant, but allows the plant volatiles to pass through as olfactory cues. These traps are economical, can be made to fit with most plant sizes, and are also reusable. Collectively, these traps will provide a solid estimate (quantifiable) of all associated community of arthropods that can also be stored for future studies.
Keywords: HerbivoresBackground
To defend against the wide range of herbivorous insects that feed on them, plants have evolved a wide range of defenses that include direct and indirect defenses (Howe and Jander, 2008; Kariyat et al., 2012 and 2013). Among these, plant volatiles are a major group that falls under indirect defenses. Plant volatiles are low molecular weight high vapor pressure organic secondary metabolites emitted by plants as part of their normal metabolic activities (Pare and Tumlinson, 1999). However, when herbivores feed on them, they alter their volatile emission by varying it both qualitatively (number of compounds emitted) and quantitatively (amount and ratio of each compound in the blend) (Pare and Tumlinson, 1999; Kariyat et al., 2012). This altered volatile bouquet is also known as herbivore-induced plant volatiles (HIPV). HIPV are the major olfactory cues that the natural enemies (predators and parasitoids) of these herbivores use as signals to find their host–the herbivores (Kariyat et al., 2012), thereby indirectly improving host plant fitness. In addition, undamaged plants also emit volatile organic compounds that are used as cues by herbivores to locate their host plant for both feeding and oviposition (Kariyat et al., 2013 and 2014). A critical component of such insect-plant interaction studies involving plant volatiles is to examine and quantify the community of insects (herbivores, predators, pollinators, and other natural enemies) that gets attracted to their hosts under field conditions (Kariyat et al., 2012). Traditionally, such studies have employed one or a combination of insect traps such as commercial sticky traps, pheromone traps, and light traps to name a few. However, most of these traps have visual aid that interferes with olfactory cues. This can affect understanding the role of olfactory cues, and in many cases, the traps only collect only a subset of the actual insect community associated with the host.
This protocol provides detailed instructions to use inexpensive and easily available materials to build a combination of three traps-which when combined will carry out comprehensive trapping of the majority of insects associated with the hosts (Kariyat et al., 2012). These traps can easily be assembled and disassembled, and are also reusable. The basic methodology involves building a cage from hardwire cloth which encloses the host plant/s of interest, two pitfall traps made of plastic cups, and a pan trap made of aluminum that fits on top of the cage. Combined, these traps will collect insects flying above and at the canopy level, and insects that either crawl or are soil dwellers.
Materials and Reagents
- Hardwire cloth, 0.635 cm mesh size, 0.61 x 3.05 m (Lowe’s, Blue Hawk, catalog number: 492388 , model: 840147)
- Zip ties, 28 cm, White,100 Ct (Lowe’s, UtilitechTM, catalog number: 76025 , model: SGY-CT18)
- Aluminum pie pans, 22.2 cm dia. x 2.9 cm (Handi-Foil, catalog number: 20305E-3 )
- Plastic cups, 9 oz, Clear (Solo, Walmart, 554949033)
- Woodenskewers/dowels, 4.76 mm dia., length 30.5 cm (Walmart, 554544726)
- White Bridal veil fabric, 0.22 m x 0.18 m, mesh size: 1 mm2 (Hobby Lobby, catalog number: 852640 )
- A4 size white paper (Staples, catalog number: 135855 , model: 135855 / 135855 WH)
- A4 size acetate sheets (Staples, catalog number: APOCG7031S , model: CG7031S)
- Glass vials 40.7ml (Fisher Scientific, catalog number: 03-338L )
- Distilled water
- Odorless tangle foot sticky glue (Tangle-Trap® Sticky Coating) (TANGLEFOOT, catalog number: 300000676 , Part No. LB8249)
- Micro 90 odorless detergent (Micro-90) (Cole-Parmer, catalog number: SK-18100-05 )
- 80% ethanol (Sigma-Aldrich, catalog number: 793191-4X1GA-PB )
Equipment
- Scissors
- Hardwire cloth wire cutter, Fatmax 5.08 cm 60 CrV snips (Stanley Black & Decker, catalog number: FMHT73563 )
- 4 °C refrigerator
- Microscope
Software
- Minitab v. 14
Procedure
Cage building and data collection workflow:
- Materials
- Assembly of hardwire cloth cage with zip ties (see Videos 1-3)
- Pan trap
- Pitfall trap
- Bridal veil and Tangle-foot
- Completed cage with pan trap and pitfall trap
- Data collection
- Data storageVideo 1. Hardwire cloth material
- After deciding on the host plant and the amount of leaf area to be used inside the trap, rollout the hardwire cloth and measure the length required to build the cage. It is advisable to build the cage in a way that the circumference of the cage is close enough to the standard size of the pie pan (see Figure 1; Videos 1-4). Then, using the equation.
Circumference (length) = 2πr (r: radius of the pan) measure the length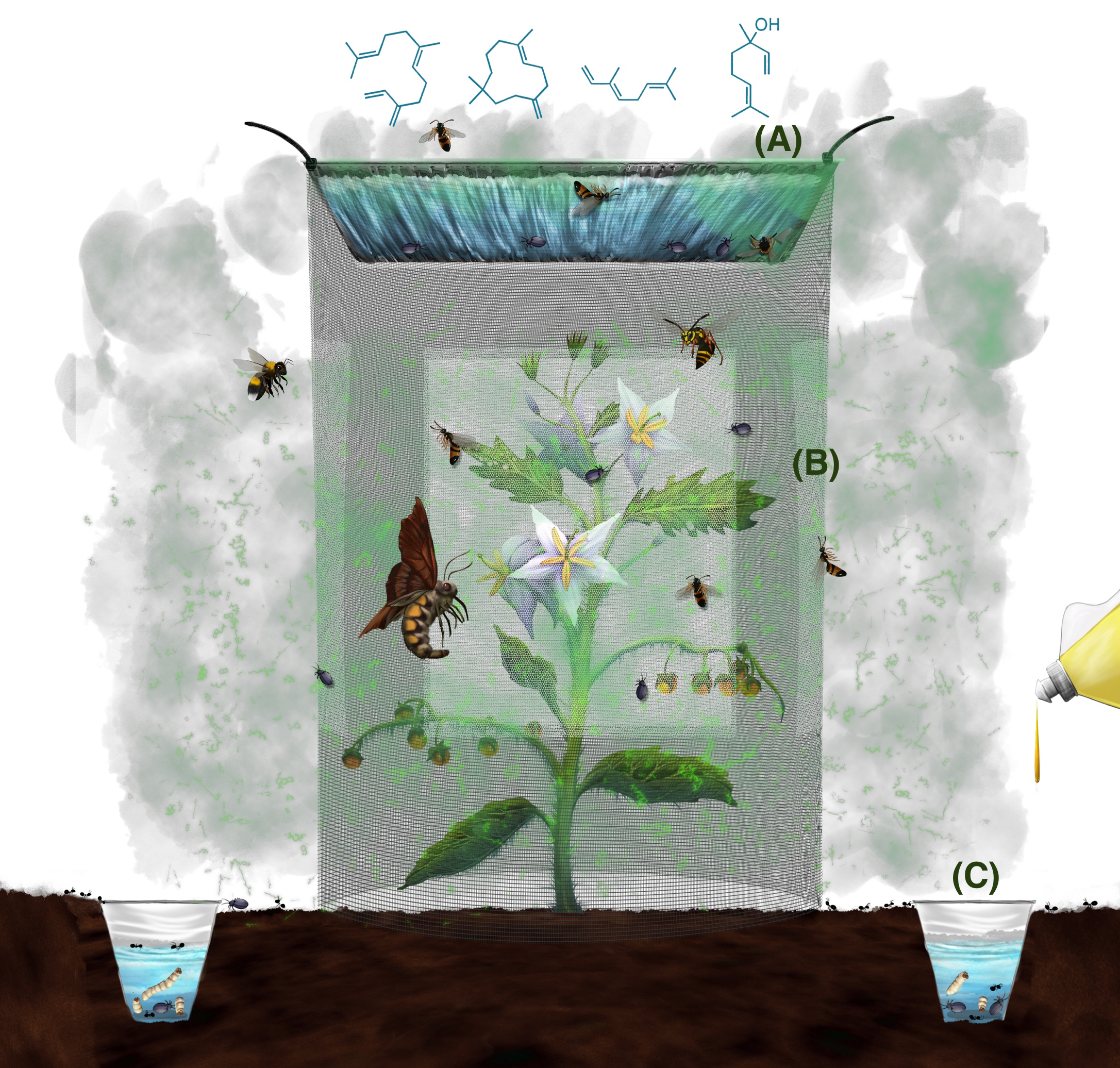
Figure 1. Field trap setup and associated insect community attracted to the traps. Volatiles produced by the plant inside the cage emits a suite of compounds that mediates the attraction of insects that mainly belong to three main guilds, the herbivores, pollinators and predators/parasitoids. Since the visual cues are masked by bridal veil sheets, the insects are attracted exclusively due to the volatile organic compounds. The three types of traps (A. pan trap, B. sticky bridal veil and C. pitfall trap) in combination, trap the soil dwelling, crawling, low and high flying insects, thereby allowing us to get a community level data set on insect abundance. (Illustration by Annette Diaz, UTRGV)Video 4. Measuring and marking the required dimensions for the cage on the hardwire cloth material - Cut the hardwire cloth into specified dimension using wire cutters. The resulting rectangle can be rolled into a cylinder (Figure 2; Videos 5 and 6).
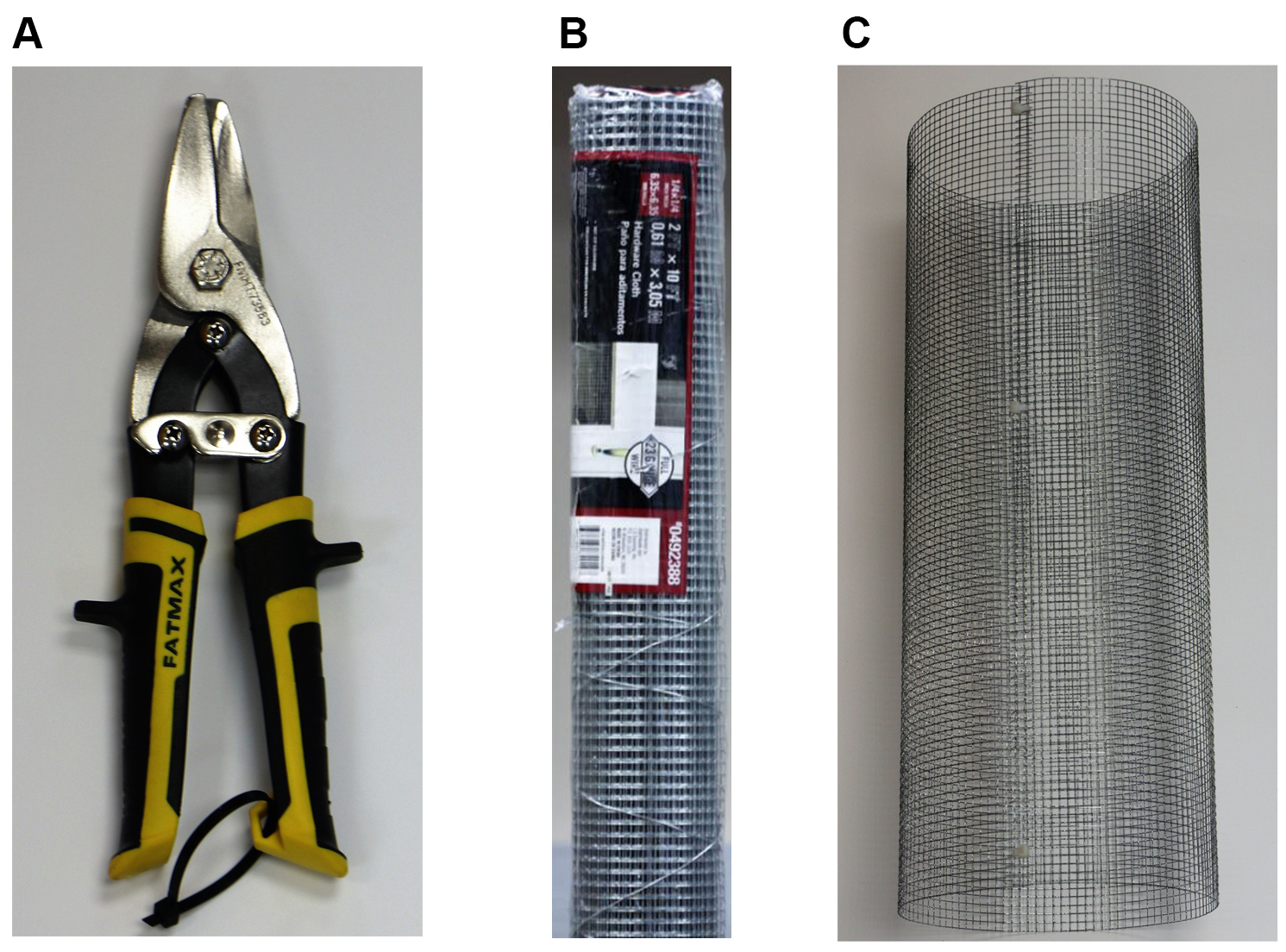
Figure 2. The cylindrical form of the rectangular hardwire cloth produced after cutting into specified dimensions using the wire cutters. A. The hardwire cutter to make cage based on the required dimension; B. Hardwire cloth material required to make the cage; C. The cylindrical form of the rectangular hardwire cloth.Video 5. Cutting the hardwire cloth material to specified dimensionsVideo 6. Making the rectangular hardware cloth material into cylindrical shape for the cage - Using zip-ties, close the cage at multiple points along the length of the cylinder (Figures 3 and 4; Videos 7 and 8), followed by snipping the zip-tie to the nub close to the cage.

Figure 3. The zip-ties for securing the wire cage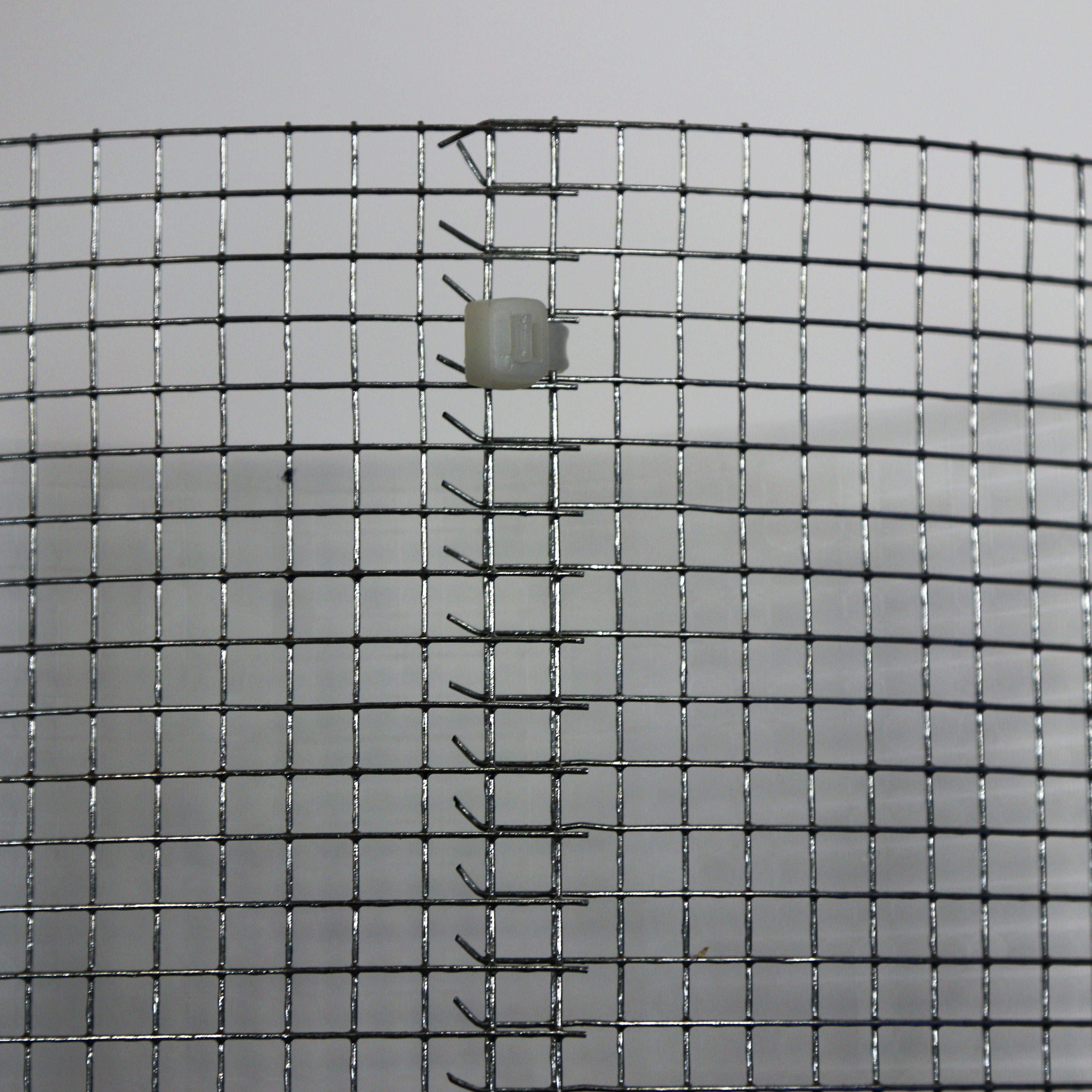
Figure 4. Close view of a zip-tie securing the ends of the hardwire cloth material cutting to make cylindrical shapeVideo 7. Securing the cylindrical frame of the cage using zip-tiesVideo 8. Removal of excess portion of the zip-ties to make the cage snug fit - Place the pie pan on top of the cage, and make 2 holes on opposite sides of the pan, and using zip ties fix it on the cage. At this point, the pan trap should sit snugly on top of the cage (Figure 1; Video 9). Pour water to 3/4th of the pan for pie pan traps, and 3/4th of the cup for pitfall traps. Add a drop of Micro-90 detergent to break the surface tension, so trapped insects won’t be able to climb out of the trap.Video 9. Demonstration of how to install the Aluminum pie pan trap on the cage frame
- Using a pair of scissors, cut three equal sized pieces of bridal veil (A4) (Figure 5). Preferred size would be 18 x 22 cm, so the sheet fits well under an A4 size paper.

Figure 5. Bridal veil material in white color to make sticky traps - Roll the veil piece into a ball, and dip generously in the odorless tangle foot glue (Figure 6).

Figure 6. Odorless Tangle-foot sticky coating/glue used to make sticky traps - Expand the bridal veil ball into a sheet, and place the three bridal veil sheets equidistant from each other on the cage along the periphery of the cage (Figures 1 and 7; Video 10).

Figure 7. Final trap setup. Final trap integrates the three bridal veil sticky traps, Aluminum-pie pan trap, and two pitfall traps to examine and quantify the insect diversity associated with the host plant inside the cage.Video 10. Steps to install bridal veil sticky trap on the cage frame - Place the cage on the plant/s of interest. If the soil is too dry, then make a depression (10 cm) along the soil surface for the cage to be fixed. In case of damp/wet soil, pushing the cage into the soil is usually enough.
- Insert two skewers each into the soil along the diameter of the cage to confirm that the cage is fixed in soil (Figure 8).

Figure 8. Wooden skewers to provide additional support to the cage against unfavorable weather conditions - On the ground, at soil level, dig two holes (around r = 4.5 cm, h = 7 cm, l = 7.3 cm) deep enough to fit two cups. The holes are to be dug diagonally opposite from each other close to the cage (see the Figure 1).
- Make sure the traps are at the soil level when the cups are placed inside the holes. Pour water upto 3/4th of the cup and add a drop of detergent (Micro-90) to break the surface tension.
- Leave the traps in the field for the duration of the experiment (48-72 h). If the experiment is exclusively looking at day/night community, use the trash cans (32 Gallon/r = 28 cm, h = 67.5 cm, Walmart Inc.) to cover the cage at desired intervals based on experimental treatments and parameters.
- After completion of the experiment, collect the pitfall traps first, pool them (if necessary), and transfer the contents to a glass vial (Fisher Scientific, 4 oz) with 80% ethanol, after draining the water.
- Repeat the same step for the pan trap on top of the cage.
- Carefully remove each bridal veil sheet from the cage (with insects stuck to them) and paste it on a new A4 paper. Immediately, place an acetate sheet on top of the bridal veil, to reduce any further damage on the insects glued to the bridal veil. The resulting file (for each cage) will have three separate data sheets, each with (a) an A4 paper layer, (b) the veil layer, and (c) the acetate sheet.
- These sheets can be stored at 4 °C until used for identification, or can be directly placed under a microscope for further examination.
- In experiments where sentinel caterpillars are left feeding (than removed for the cage experiment), the cage has to be modified to restrict any entry of parasitoid wasps inside the cage. To accomplish this, use 1/8th of an inch mesh size (0.3 x 0.3 cm2).
Data analysis
- Classification: Depending on the objectives of the experiment, the insects can be identified in order–when possible by family, genus, and species–and can then be classified based on phylogeny and feeding guild (e.g., sucking and piercing, chewing and lapping, siphoning; Kariyat et al., 2012). For experiments on herbivory and trophic interactions, the methodology is as follows: winged Hymenoptera (parasitoids and predatory wasps), ants; generalist and specialist coleopteran herbivores, lepidoptera (caterpillars and adults), predatory coleopterans (e.g., Coccinellidae); dipterans, aphids, and ants. The data from all the three traps per cage (pit fall traps, one pan trap, and three bridal veil sheets) can also be pooled to examine the whole community associated with the host plant (Kariyat et al., 2012).
- Statistics: Multivariate statistics using MANOVA is a commonly used method to analyze such data sets using the insect guilds as variables (Statistical software Minitab v. 14; Kariyat et al., 2012). Insect count data for each variable will have to be transformed (e.g., rank transformation since it is count data) prior to analysis. After MANOVA analyses, these variables can be subjected to univariate ANOVA’s, with reduced models that included the factors and interaction terms identified as being significant in the overall MANOVA.
Notes
This protocol provides an easy and inexpensive method to collect insect community data, with minimum damage to the specimens. In addition, unlike many other traps, rain, wind and dust will only cause minimum damage. The sticky glue tanglefoot is water resistant and retains its stickiness even in rain. The traps can be covered using trashcans to specifically target times of collection (e.g., day/night separation, active herbivore/predator feeding times), and because they are made of metal wire, the main trap is also reusable. The protocol can also be modified based on what the questions are, and the type of host plants by altering the size and shape of the cage to fit plants of different growth patterns. The same cage can also be dismantled and reshaped based on the need. The bridal veil sheets on acetate will stay preserved for years at 4 °C and can be stored in relatively small space. Like any other field trapping/ecology protocol, this set is also prone to variability, mainly due to the field conditions. Additional soil reinforcement might be warranted based on landscape and slope. Care should be taken to ensure that the aluminum pie pan, and pitfall traps do not overflow in rain, or get muddy.
Acknowledgments
We thank Drs. Andrew Stephenson and Mark Mescher for helping with the design of the cage. We also thank Dr. Kerry Mauck for help with the statistics for the primary manuscript. We also acknowledge many other labs and personnel who use similar type of cages that we made the design from. We also thank Annette Diaz for the illustration. This work was funded by the UTRGV strategic plan award, and startup funds to Dr. Rupesh Kariyat.
A brief version of the protocol was published in Kariyat et al., 2012 [Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.)].
Competing interests
The authors declare that there are no conflicting and/or competing interests.
References
- Howe, G. A. and Jander, G. (2008). Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41-66.
- Kariyat, R. R., Mauck, K. E., De Moraes, C. M., Stephenson, A. G. and Mescher, M. C. (2012). Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecol Lett 15(4): 301-309.
- Kariyat, R. R., Mauck, K. E., Balogh, C. M., Stephenson, A. G., Mescher, M. C. and De Moraes, C. M. (2013). Inbreeding in horsenettle (Solanum carolinense) alters night-time volatile emissions that guide oviposition by Manduca sexta moths. Proc Biol Sci 280(1757): 20130020.
- Kariyat, R. R., Scanlon, S. R., Moraski, R. P., Stephenson, A. G., Mescher, M. C. and De Moraes, C. M. (2014). Plant inbreeding and prior herbivory influence the attraction of caterpillars (Manduca sexta) to odors of the host plant Solanum carolinense (Solanaceae). Am J Bot 101(2): 376-380.
- Pare, P. W. and Tumlinson, J. H. (1999). Plant volatiles as a defense against insect herbivores. Plant Physiol 121(2): 325-332.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kariyat, R., Chavana, J. and Kaur, J. (2018). An Inexpensive and Comprehensive Method to Examine and Quantify Field Insect Community Influenced by Host Plant Olfactory Cues. Bio-protocol 8(16): e2967. DOI: 10.21769/BioProtoc.2967.
Category
Plant Science > Plant biochemistry > Other compound
Environmental science > Plant > Plant-insect interaction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










