- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
BMV Propagation, Extraction and Purification Using Chromatographic Methods
Published: Vol 8, Iss 14, Jul 20, 2018 DOI: 10.21769/BioProtoc.2935 Views: 6897
Reviewed by: Vamseedhar Rayaprolu Joanna Sztuba-SolinskaJolene Ramsey

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Inducible HIV-1 Reservoir Reduction Assay (HIVRRA), a Fast and Sensitive Assay to Test Cytotoxicity and Potency of Cure Strategies to Reduce the Replication-Competent HIV-1 Reservoir in Ex Vivo PBMCs
Jade Jansen [...] Neeltje A. Kootstra
Jul 20, 2025 2458 Views

Assembly and Mutagenesis of Human Coronavirus OC43 Genomes in Yeast via Transformation-Associated Recombination
Brett A. Duguay and Craig McCormick
Aug 20, 2025 3035 Views
Abstract
Brome mosaic virus (BMV) is a well-known plant virus representing single-stranded RNA (ssRNA) positive-sense viruses. It has been widely used as a model in multiple studies concerning plant virus biology, epidemiology and the application of viral capsids in nanotechnology. Herein, we describe a method for BMV purification based on ion-exchange and size-exclusion chromatography. The presented method is of similar efficiency to previously described protocols relying on differential centrifugation and can easily be scaled up. The resulting BMV capsids are stable and monodisperse and can be used for further applications.
Keywords: Brome mosaic virusBackground
One of the key challenges for nanotechnology to overcome is elaboration of effective and tissue-specific drug delivery methods. Plant viruses and virus-like particles (VLPs) are biocompatible and biodegradable and do not contain pathogens hazardous to human or animal health, and are a safe alternative to the synthetic drug carriers which often activate an undesirable response of the immune system or accumulate in the body to toxic levels. Finally, the production of the viral capsids is relatively cheap and fast (Ren et al., 2007; Arcangeli et al., 2014).
Brome mosaic virus (BMV) of the Bromoviridae family is a good candidate for use as a nanoparticle carrier since it shows all of the abovementioned features and is one of the best-studied plant viruses (Figlerowicz, 2000; Alejska et al., 2005; Urbanowicz et al., 2005; Wierzchoslawski et al., 2006; Kao et al., 2011). It is a positive-sense RNA virus with a genome composed of three different RNA segments. Each genomic RNA is packed into a separate capsid. The capsids are morphologically indistinguishable although they differ with their biophysical and biological properties. The molecular weight of the BMV virion is 4.6 MDa, and its diameter is approximately 28 nm. The BMV capsid has a T = 3 icosahedral construction and is comprised of 180 19.4-kDa CP monomers (Ni et al., 2014; Vaughan et al., 2014).
Although a commercial usage of VLPs as drug carriers is a distant future goal, BMV-based VLPs have already been loaded with various nanoparticles. The most effective VLP formation was obtained when gold nanoparticles were coated with polyanions, such as carboxylated polyethylene glycol. However other nanoparticles, such as spherical and cubic iron oxide were also encapsidated in BMV-based VLPs (Dragnea et al., 2003; Chen et al., 2006; Huang et al., 2011; Guerrero et al., 2017). BMV capsids carrying quantum dots might find an application as luminescent bioprobes (Dixit et al., 2006). In addition, the encapsulation of a chromophore, indocyanine green, into empty BMV capsids has also been archived (Jung et al., 2011). All previous reports described BMV preparations that were purified by differential ultracentrifugation using sucrose or cesium chloride gradients. These methods, although generate excellent quality viral preparations, have quantitative limitations. In this protocol we describe an efficient (up to 0.2 mg of virus from 1 g of plant tissue), chromatography-based method of obtaining BMV of high purity and quality; this method is an easy alternative to existing methods. The produced BMV capsids show high monodispersity and structure-environment dependency, features that are crucial for the formation of functional VLPs (Strugala et al., 2017) (Figure 2). Similarly to previously described methods, our procedure can be applicable to the purification of other plant viruses of similar capsid size. For example, it was highly efficient for the purification of the red clover necrotic virus (RCNMV) and resulted with monodisperse viral preparations. Finally, our protocol might be easily adapted for larger-scale purification.
Materials and Reagents
Notes:
- Regarding the materials, reagents and equipment, a proper and comparable setup may be used.
- All prepared buffers should be filtered through a 0.45 μm filter. Additionally, buffers for Size Exclusion Chromatography should be degassed (Degassing process takes 1 h for 1 L buffer. Store degassed buffers at 4 °C, for 1 month).
- BMV propagation
- Pots (Floser, catalog number: BTS 10,5 ), H 80 mm, Ø 105 mm, vol. 0.46 L
- Garden trays, 60 cm Square Tray Black (Garland Products, catalog number: G191B )
- Soil (PPHU Socha, all-purpose garden soil pH 5.5-6.5), quartz sand (PPHU Socha, 1mm diameter)
- Gloves (Mercator medical, Nitrylex PF classic)
- Tips
5,000 μl (PZ HTL, catalog number: 35001 )
1,000 μl (OMNITIP, catalog number: 85710 )
200 μl (OMNITIP, catalog number: 83710 )
10 μl (OMNITIP, catalog number: 81710 ) - Barley (Hordeum vulgare) seeds
- BMV-infected plants (barley or Chenopodium quinoa)
- Carborundum F400 (KREMER POLSKA, catalog number: 58750 )
- Liquid nitrogen (Air Products, CryoEase)
- Sodium phosphate monobasic (Sigma-Aldrich, catalog number: S3139 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670 )
- Inoculation buffer (see Recipes)
- Pots (Floser, catalog number: BTS 10,5 ), H 80 mm, Ø 105 mm, vol. 0.46 L
- BMV isolation from plants
- NalgeneTM Oak Ridge High-Speed PPCO centrifuge tube (Thermo Fisher Scientific, catalog number: 3119-0050 )
- Tips (see Materials and Reagents A5)
- NalgeneTM Polysulfone reusable bottle top filter, 500 ml, collar 45 mm (Thermo Fisher Scientific, catalog number: DS0320-5045 )
- Filters 0.45 μm, 47 mm (Merck, catalog number: HAWG047S6 )
- Liquid nitrogen or dry ice
- Sodium acetate (CH3COONa) (Sigma-Aldrich, catalog number: S2889 )
- Boric acid (H3BO3) (MP Biomedicals, catalog number: 194810 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670 )
- Chloroform (Firma Chempur, catalog number: CHEM*112344305 )
- 30% polyethylene glycol 8000 (PEG 8000) (BioShop, catalog number: PEG800 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S3264 )
- BMV extraction buffer (see Recipes)
- 10x L buffer (phosphate buffer) (see Recipes)
- NalgeneTM Oak Ridge High-Speed PPCO centrifuge tube (Thermo Fisher Scientific, catalog number: 3119-0050 )
- BMV purification
- Discardit IITM 5 ml syringe (BD, catalog number: 309050 )
- 50 ml conical tubes (SARSTEDT, catalog number: 62.548.004 )
- Tips (see Materials and Reagents A5)
- Micro tubes 1.5 ml (SARSTEDT, catalog number: 72.690.001 )
- Amicon Ultra-15 Filters (Merck, catalog number: UFC910024 )
- Filters 0.45 μm, 47 mm (Merck, catalog number: HAWG047S6 )
- PP centrifuge tubes 12 x 75 mm (Bionovo, catalog number: E-1649 )
- Millex-HV Syringe Driven Filter Unit (Merck, Millex Filter, catalog number: SLHV013NL )
- DEAE-cellulose (Sigma-Aldrich, catalog number: D3764 )
- Sodium chloride (NaCl) (BioShop, catalog number: SOD001 )
- Trizma base (Sigma-Aldrich, catalog number: T1503 )
- Glycerol (Carl Roth, Rotipuran, catalog number: 3783 )
- Phosphate-buffered saline (PBS) (Thermo Fisher Scientific, catalog number: 18912014 )
- Discardit IITM 5 ml syringe (BD, catalog number: 309050 )
- BMV analysis
- Micro tubes 1.5 ml (SARSTEDT, catalog number: 72.690.001 )
- Tips (see Materials and Reagents A5)
- Quartz cuvette (Hellma, catalog numbers: 105.201-QS , 105.231-QS )
- 4-20% Mini-PROTEAN® TGXTM Precast Protein Gels, 10-well, 30 μl (optional, Bio-Rad Laboratories, catalog number: 4561093 )
- Rotiphorese NF-acrylamid/bis 19:1 (Carl Roth, catalog number: A516.1 )
- Sodium dodecyl sulfate (SDS) (Carl Roth, catalog number: 0183.2 )
- Ammonium persulfate (APS) (Sigma-Aldrich, catalog number: A3678 )
- TEMED (BioShop, catalog number: TEM001.50 )
- Perfect Tricolor Protein Ladder (EURx, catalog number: E3210-01 )
- SDS-PAGE sample loading buffer: NovexTM Tris-Glycine SDS Sample Buffer (2x) (Thermo Fisher Scientific, catalog number: LC2676 )
- PageBlueTM Protein Staining Solution (Thermo Fisher Scientific, catalog number: 24620 )
- Micro tubes 1.5 ml (SARSTEDT, catalog number: 72.690.001 )
Equipment
- Preparing Buffers–all the steps:
- Bottles
1 L (Kavalierglass, Simax, catalog number: 1632414321940 )
500 ml (Kavalierglass, Simax, catalog number: 1632414321500 )
250 ml (Kavalierglass, Simax, catalog number: 1632414321250 ) - Cylinders
500 ml (Kavalierglass, Simax, catalog number: 1632432111343 )
250 ml (Kavalierglass, Simax, catalog number: 1632432111238 )
100 ml (Kavalierglass, Simax, catalog number: 1632432111130 )
- Bottles
- BMV propagation
- Beakers
1 L (Kavalierglass, Simax, catalog number: 1632417010940 )
800 ml (Kavalierglass, Simax, catalog number: 1632417010800 )
600 ml (Kavalierglass, Simax, catalog number: 1632417010600 )
400 ml (Kavalierglass, Simax, catalog number: 1632417010400 )
250 ml (Kavalierglass, Simax, catalog number: 1632417010250 )
100 ml (Kavalierglass, Simax, catalog number: 1632417010100 ) - Ice bucket (Round Ice Bucket with Lid, 4 L) (Corning, catalog number: 432122 )
- Porcelain unglazed mortar (Conbest, catalog number: 891-03-220 ) and porcelain unglazed pestle (Conbest, catalog number: 892-03-135 )
- Pipettes
- Eppendorf Research® plus 0.5-5 ml (Eppendorf, model: Research® plus , catalog number: 3123000071)
- Discovery comfort DV1000 (PZ HTL, catalog number: 4046-DV )
- Discovery comfort DV100 (PZ HTL, catalog number: 4044-DV )
- Discovery comfort DV10 (PZ HTL, catalog number: 4042-DV )
- Discovery comfort DV2 (PZ HTL, catalog number: 4041-DV )
- Eppendorf Research® plus 0.5-5 ml (Eppendorf, model: Research® plus , catalog number: 3123000071)
- Fitotron® plant growth chamber (Percival Scientific, model: E41-L2 )
- Beakers
- BMV extraction from plants
- Pipettes (see Equipment B4)
- Porcelain unglazed mortar (Conbest, catalog number: 891-03-220 ) and porcelain unglazed pestle (Conbest, catalog number: 892-03-135 )
- Vortex (Reax control) (Heidolph Instruments, catalog number: 541-11000-00 )
- Centrifuge (Eppendorf, models: 5415 R , 5810 R )
- Laboratory scale (RADWAG Balances and Scales, model: PS 1000.R2 )
- IKA MS 3 digital shaker (IKA, model: MS 3 )
- Pipettes (see Equipment B4)
- BMV purification
- Barnstead GenPure LifeScience UV/UF (TKA Wasseraufbereitungssysteme, catalog number: 08.2204 )
- Versatile laboratory pump (PL 2/1) (AGA LABOR, model: Basic 36 )
- Ion Exchange Chromatography
- Peristaltic pump (Masterflex L/S, Easy Load II Head, Cole-Parmer, catalog number: EW-77200-50 )
- CrystalCruz® chromatography column 2.5 x 10 cm (Santa Cruz Biotechnology, catalog number: sc-205558 )
- Pipettes (see Equipment B4)
- Peristaltic pump (Masterflex L/S, Easy Load II Head, Cole-Parmer, catalog number: EW-77200-50 )
- Size-Exclusion Chromatography (SEC)
- HiPrep 16/60 Sephacryl S-500 HR (GE Healthcare, catalog number: 28-9356-06 )
- ÄKTAprime plus (GE Healthcare)
- HiPrep 16/60 Sephacryl S-500 HR (GE Healthcare, catalog number: 28-9356-06 )
- Barnstead GenPure LifeScience UV/UF (TKA Wasseraufbereitungssysteme, catalog number: 08.2204 )
- BMV analysis
- Concentration measurement
- Pipettes (see Equipment B4)
- Apparatus for SDS Polyacrylamide gel electrophoresis (PAGE) (Mini-PROTEAN® Tetra Vertical Electrophoresis Cell) (Bio-Rad Laboratories, catalog number: 1658004 )
- Mini-PROTEAN® Tetra Cell Casting Module (Bio-Rad Laboratories, catalog number: 1658013 )
- Power supply (Wealtec, model: ELITE 300 Plus )
- Multi Bio 3D (Biosan, catalog number: BS-010125 )
- Thermoblock (Biosan, model: Bio TDB-100 )
- BioPhotometer (Eppendorf, catalog number: 550507804 )
- Pipettes (see Equipment B4)
- DLS analysis
- Pipettes (see Equipment B4)
- Malvern Zetasizer μV (Malvern Instruments)
- Pipettes (see Equipment B4)
- Concentration measurement
Procedure
Note: To prepare BMV inoculum, previously infected barley or Chenopodium have to be available.
- BMV propagation in Hordeum vulgare
- Set up a set of pots (25-50 pots) filled with soil mixture (mix garden soil:sand, 1:5).
- Plant about 10 Hordeum vulgare seeds per pot, cover with a 1 cm layer of soil and water the seeds.
- Place the garden tray with pots in the Fitotron® plant growth chamber (22 °C, 40 % humidity and 16/8 photoperiod, light intensity 600 μmol m-2 sec-1)
- Water the plants every 2-3 days for 2 weeks.
- Inoculate Hordeum vulgare plants 2 weeks after seeding (plants should have just one leaf):
- Grind 1 g of previously infected Chenopodium or barley leaves with visible infection symptoms (white or pale yellow dots in Chenopodium or white or pale green mosaic in barley) with a mortar and pestle. It is important to keep low temperature during grinding and to chill the mortar and pestle with liquid nitrogen before and during grinding (every 2-3 min). After grinding place on ice. Add 2 ml of inoculation buffer and mix with the pestle. Ground leaf solution is a green liquid with small, visible bits.
- Dust young plant leaves with carborundum.
- Put 10 μl of BMV suspension from Step A5a on every young barley leaf and rub in gently with a gloved hand all over the leaf (beware of crushing the leaves).
- Water the plants once every 2-3 days.
- Grind 1 g of previously infected Chenopodium or barley leaves with visible infection symptoms (white or pale yellow dots in Chenopodium or white or pale green mosaic in barley) with a mortar and pestle. It is important to keep low temperature during grinding and to chill the mortar and pestle with liquid nitrogen before and during grinding (every 2-3 min). After grinding place on ice. Add 2 ml of inoculation buffer and mix with the pestle. Ground leaf solution is a green liquid with small, visible bits.
- Two weeks after inoculation harvest all infected leaves (with visible white or pale green mosaic), divide into 5 g aliquots, wrap in aluminum foil and freeze in liquid nitrogen, store at -80 °C.
- Set up a set of pots (25-50 pots) filled with soil mixture (mix garden soil:sand, 1:5).
- BMV extraction from plants
- Place frozen leaves in a mortar and grind the tissue with the pestle until they become powder. Use liquid nitrogen (preferred) or dry ice to keep plant tissue frozen (pour liquid nitrogen directly into the mortar over 2-3 min).
- Add BMV extraction buffer to crushed tissue (4 ml of buffer per 1 g of plant tissue).
- Transfer the virus suspension with an appropriate pipette into PPCO centrifuge tubes. Take as much suspension as possible.
- Place the PPCO centrifuge tubes with BMV suspension on ice. Add 1/5th volume chloroform to the suspension.
- Vortex for 30 sec, 2,500 rpm.
- Centrifuge for 5 min at 4 °C, 5,000 x g.
- Transfer the supernatant into a new PPCO centrifuge tube (be careful, not touch the pellet; discard the precipitate), and add 1/3rd volume 30% PEG (water solution). Vortex for 30 sec, 2,500 rpm.
- Incubate on ice for 30 min without shaking.
- Centrifuge for 15 min at 4 °C, 12,000 x g.
- Discard supernatant. Suspend the precipitate in 2 ml of cold 1x L buffer and shake it using vortex overnight, 4 °C, 750 rpm.
- The BMV suspension can be stored for one week at 4 °C.
- Place frozen leaves in a mortar and grind the tissue with the pestle until they become powder. Use liquid nitrogen (preferred) or dry ice to keep plant tissue frozen (pour liquid nitrogen directly into the mortar over 2-3 min).
- BMV purification
- Ion Exchange Chromatography
- To the sample from Step B10 add glycerol to 5% final concentration. Mix the sample and place it on ice.
- Prepare DEAE cellulose.
- Suspend 10 ml of DEAE cellulose in deionized water.
- Pour DEAE cellulose in CrystalCruz® chromatography column.
- Attach the column to a peristaltic pump.
- Settle the DEAE cellulose with the 0.5 ml/min flow rate. Discard the flow through.
- Wash DEAE cellulose with 5 volumes of deionized water at a flow rate of 0.5 ml/min.
- Wash the DEAE cellulose with 2 volumes of 1x L buffer. Don’t let cellulose dry. Leave a 2 mm layer of buffer over DEAE cellulose surface.
- Suspend 10 ml of DEAE cellulose in deionized water.
- Place the sample from Step C1a on DEAE cellulose.
- Filter the BMV sample through DEAE cellulose with 0.2 ml/min flow rate.
- Wash DEAE cellulose with NaCl gradient (0.05 M, 0.1 M, 0.2 M, 0.3 M, 0.4 M and 0.5 M) in 1x L buffer. Use 20 ml of buffer per 1 fraction. Collect all the flow through volume. Store at 4 °C up to one week.
- Place 50 μl from each fraction in Eppendorf tubes, add SDS-PAGE sample loading buffer, denature at 95 °C in thermoblock for 5 min and separate by SDS-PAGE to confirm the presence of BMV virions (see Step D1). The exemplary SDS-PAGE result is shown in Figure 1.
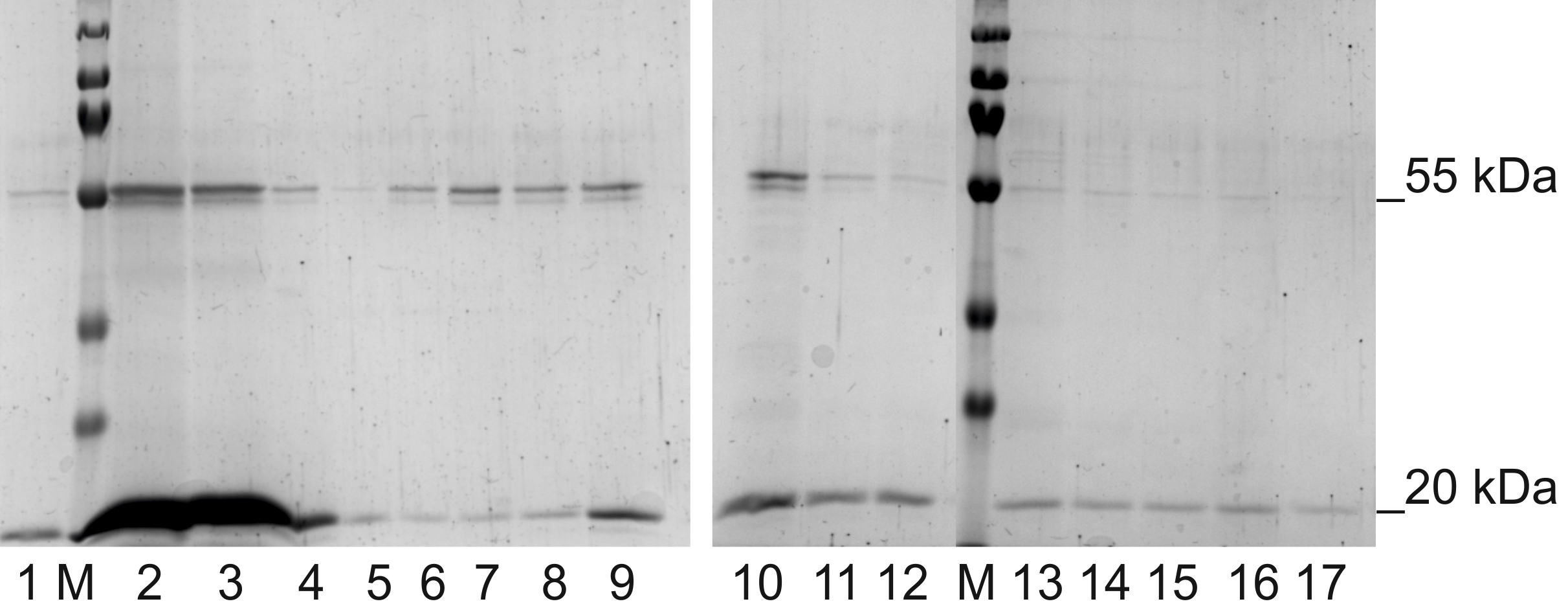
Figure 1. BMV after ion-exchange chromatography with NaCl gradient and separation by SDS-PAGE. The particular fractions eluted with NaCl gradient are as follows: 0.05 M NaCl–lanes 1-3, 0.1 M NaCl–lanes 4-6, 0.2 M NaCl–lanes 7-9, 0.3 M NaCl–lanes 10-12, 0.4 M NaCl –lanes 13-15 and 0.5 M NaCl–lanes 16-17, lane marked with M refers to Protein tricolor ladder). The 20 kDa band represents BMV capsid protein, while 55 kDa band represents protein contamination. To obtain pure BMV, fractions with the highest concentration of the virus (2 and 3) were concentrated and subjected to SEC. - Combine BMV-containing fractions and concentrate to 2 ml by centrifugation in Amicon Ultra-15 Filters at 4 °C, 4,700 x g (Figure 2).
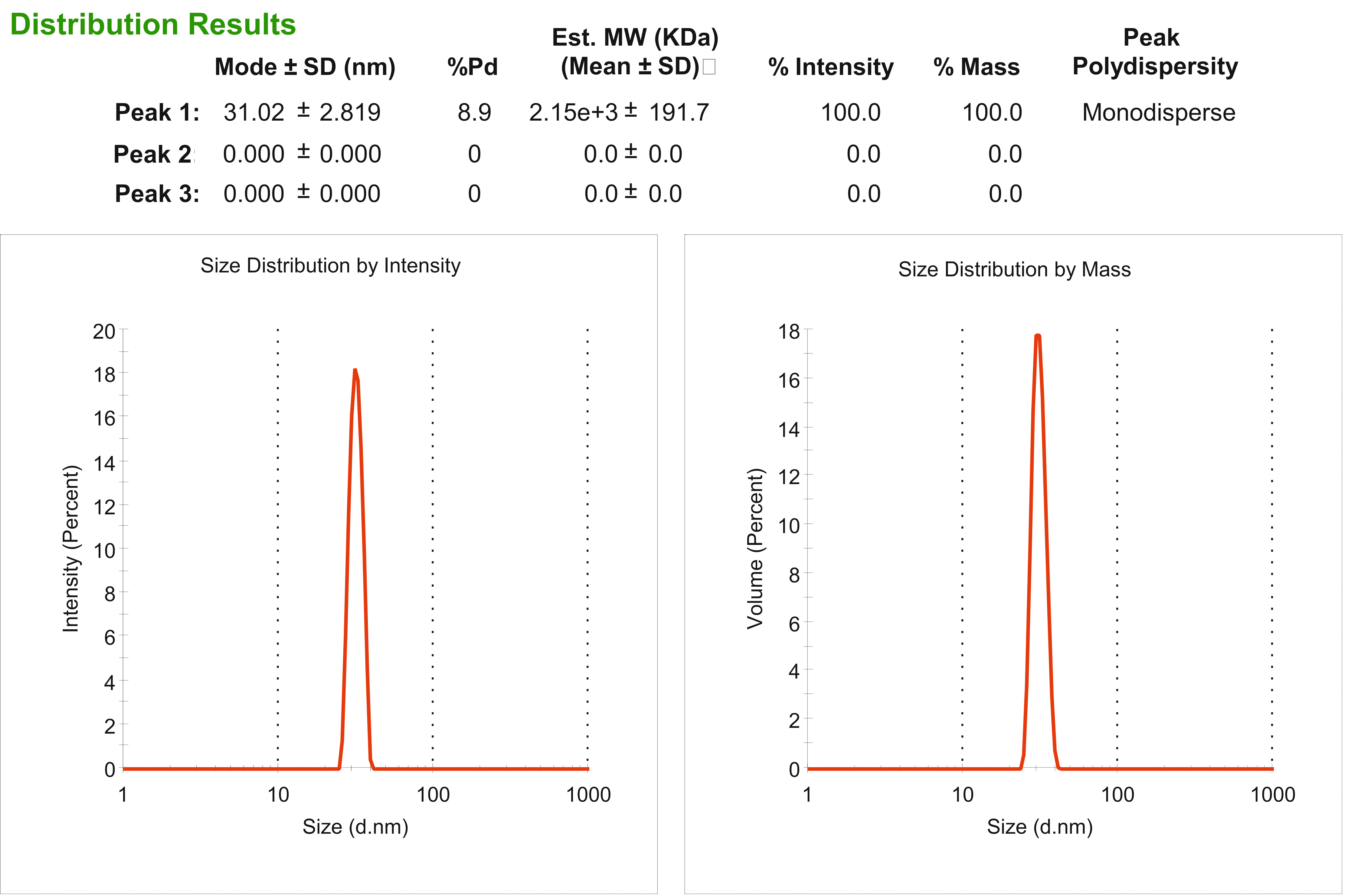
Figure 2. DLS results recorded for BMV preparation after the complete purification procedure. The sample is 100% monodisperse and contains the particles with a diameter of approximately 31 nm which corresponds to BMV dimensions.
- To the sample from Step B10 add glycerol to 5% final concentration. Mix the sample and place it on ice.
- Size-Exclusion Chromatography (SEC)
- All buffers used for SEC should be filtered through 0.45 μm membrane and degassed.
- Use HiPrep 16/60 Sephacryl S-500 High Resolution column connected to the ÄKTAprime plus system to purify BMV virions. When not in use, column should be filled with 20% ethanol. All buffers used for SEC should be filtered through 0.45 μm membrane and degassed. All runs should be performed at max pressure limit of 0.5 MPa.
- Wash the sample loop with at least two sample loop volumes of the deionized water.
- Remove ethanol by washing the column with 130 ml degassed, deionized water (0.5 ml/min flow rate) and 130 ml of degassed PBS 1x (0.5 ml/min flow rate). The volume of 130 ml refers to 1 volume of the bed (120 ml) and 10 ml of column equilibration volume.
- Set the purification run according to Table 1:
Table 1. ÄKTAprime Method Breakpoints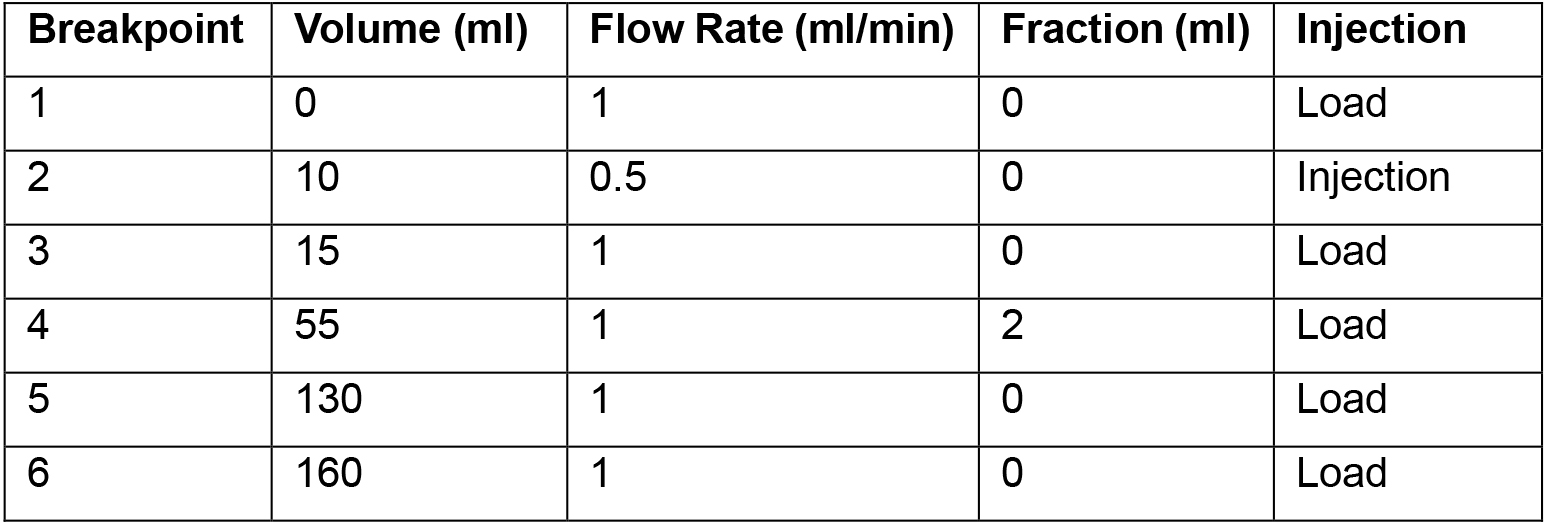
- Load the concentrated BMV sample from Step C1g on the ÄKTAprime plus system, connected to HiPrep 16/60 Sephacryl S-500 High Resolution. Sample volume should not be over 4% of the bed volume–4.8 ml. Load the sample using a syringe and a 0.45 μm filter. Run purification at 4 °C. (Figure 3)
- Place all the fractions from SEC at 4 °C.
- Rinse the sample loop with 5 loop volumes of deionized water.
- Wash the HiPrep 16/60 Sephacryl S-500 High Resolution column with 180 ml deionized, degassed water and 180 ml of degassed 20% ethanol.
- Place 50 μl from each fraction containing protein (according to the SEC chromatogram) in Eppendorf tubes, add SDS-PAGE sample loading buffer, denature at 95 °C and separate by SDS-PAGE to confirm the presence of BMV virions (see Step D1).
- Collect BMV-containing fractions and concentrate to 1 ml using Amicon Ultra-15 Filters.
- For storing, freeze in liquid nitrogen, store at -80 °C.
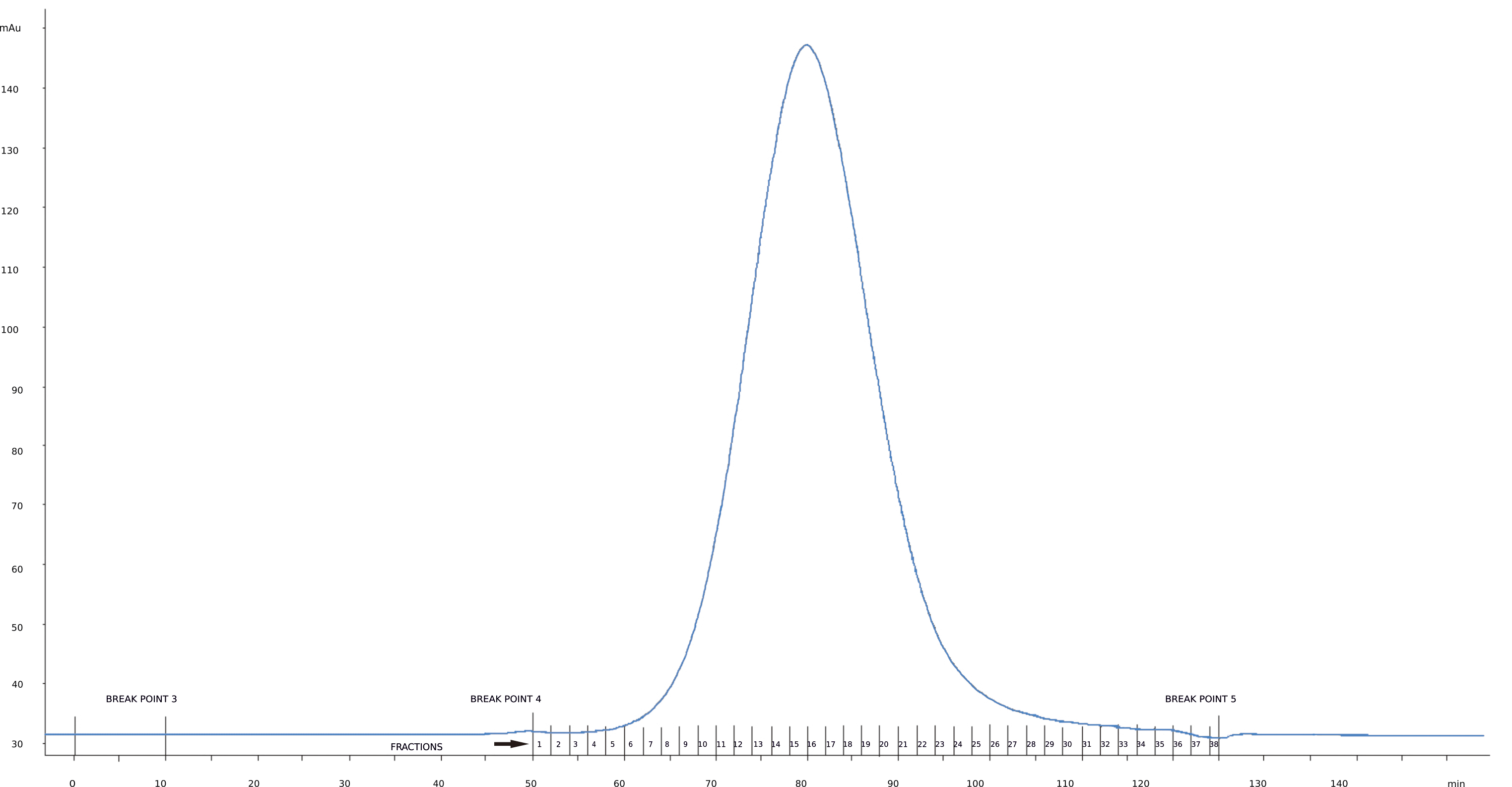
Figure 3. Size exclusion chromatogram recorded for BMV. Horizontal axis represents fractions collected (red numbers correspond to the fractions numbers) while vertical axis corresponds to UV280 absorbance (arbitrary units). The blue curve with single peak is the chromatographic plot. Fractions covered by the peak contain the virus (see Figure 4).
- All buffers used for SEC should be filtered through 0.45 μm membrane and degassed.
- Ion Exchange Chromatography
- BMV analysis
- SDS PAGE
- Gel preparation
Prepare separating gel- Set the glass plates in the casting frame (100.0 mm x 83.0 mm x 1.0 mm spacer).
- Prepare separating gel solution according to Table 2 in a beaker or 50 ml conical tube. TEMED must be added just before pouring the gel between the glass plates.
- Mix the gel solution and pour with a pipette 4 ml of the solution between the glass plates (1.0 mm spacer).
- Pour 0.5 ml of deionized water or isopropanol with a pipette on the top of gel.
- Wait 20 min till gel polymerizes.
- Prepare the stacking gel solution according to Table 2 in a beaker or 50 ml conical tube. TEMED must be added just before pouring the gel between the glass plates.
- Discard the water/isopropanol from the top of separating gel. Dry the space above the separating gel with a tissue.
- Mix the gel solution and pour between the glass plates.
- Place an appropriate comb (see Equipment E1c). Avoid trapping air under the comb.
- Wait 20 min till gel polymerizes.
Table 2. SDS Polyacrylamide Gel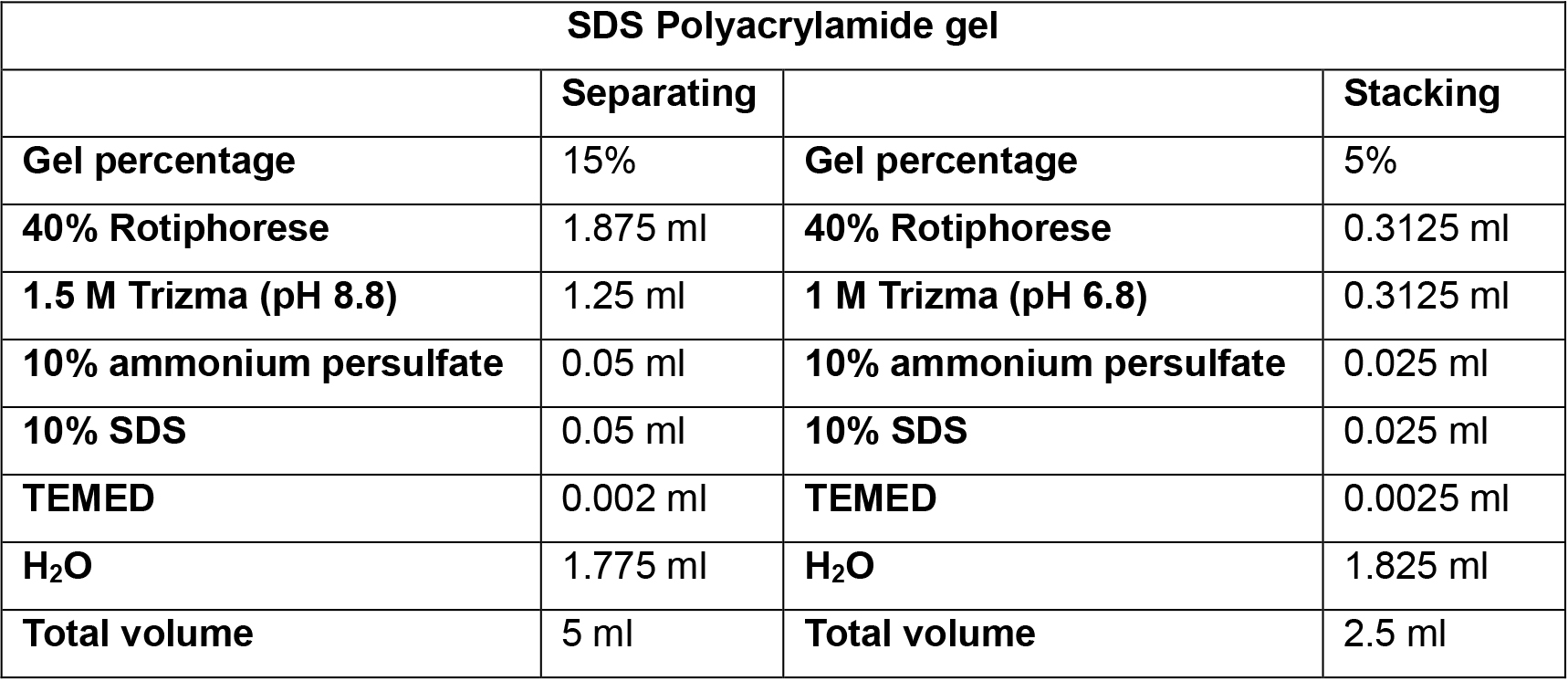
- Set the glass plates in the casting frame (100.0 mm x 83.0 mm x 1.0 mm spacer).
- Prepare the samples. Place 50 μl of protein solutions in Eppendorf tubes, add SDS-PAGE sample loading buffer. Denature at 95 °C for 10 min.
- Take out the comb and place the glass plates with gel in the electrophoresis apparatus and fill the inner and outer chamber with electrophoresis buffer.
- Load 10 μl of the sample into each well. Load 5 μl of the Protein tricolor ladder to one of the wells.
- Perform electrophoresis:
Voltage: 200 V
Expected current: initial 25-50 mA, final 20-31 mA
Runtime: 30-40 min (end the run, when the dye runs to the end of the gel)
Temperature: room temperature - End the run. Open the cassette. Using a lever separate the glass plates and gently take out the gel.
- Place the gel in a plastic box filled with deionized water. Heat the gel in a microwave for 1 min. Do not boil. Place the box on the Multi Bio 3D and stir at 50 rpm, 3D Reciprocal motion 01 (360°), Vibro type motion off, for 5 min. Change water and repeat heating and stirring 2 times.
- Place the gel in PageBlue Protein Staining Solution. Heat in a microwave for 1 min. Do not boil. Stir the gel on the shaker for 30 min.
- Discard the PageBlue Protein Staining Solution. Wash the gel in the deionized water. Observe the protein bands (Figure 4).
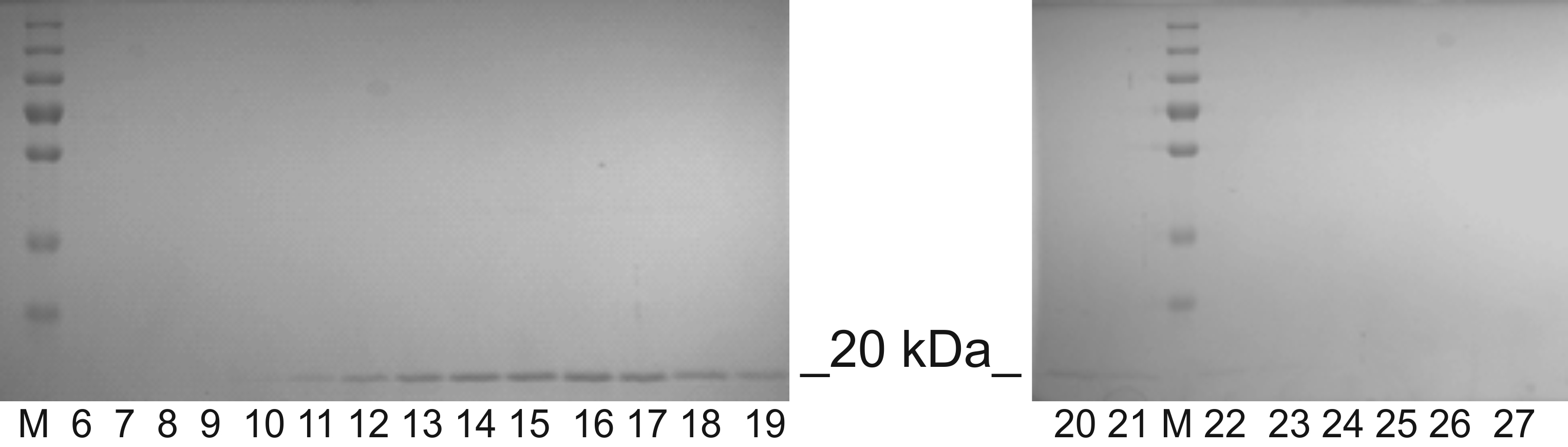
Figure 4. BMV after SEC and separation by SDS-PAGE. Lanes M–Protein tricolor ladder, lanes 6-27 refer to corresponding fractions from SEC chromatogram (see Figure 3).
- Gel preparation
- BMV concentration
- Use the BioPhotometer (Eppendorf) and quartz cuvette (Hellma) to measure the BMV concentration. Perform RNA measurement.
- Use 100 μl of PBS 1x as a blank.
- Dilute BMV sample from Step C2k with PBS 10-fold (1 vol. of BMV sample to 9 vol. of PBS 1x).
- Measurement of BMV concentration is performed at 260 nm wavelength.
- Assess BMV concentration according to the equation:
Cv = A260/ε*L
where,
Cv–virus concentration [mg/ml]
A260–absorbance at 260 nm
εBMV = 5.15 [cm-1 mg-1 ml] (Bockstahler et al., 1962)
L = 1 [cm] (cuvette path - length)
- Use the BioPhotometer (Eppendorf) and quartz cuvette (Hellma) to measure the BMV concentration. Perform RNA measurement.
- DLS analysis
- Perform DLS analysis using Zetasizer μV and a 1-cm-path-length quartz cuvette (Hellma). Clean the cuvette with deionized water 3 times. Dry the cuvette. Place 4 μl of the BMV sample in the quartz cuvette.
- Perform DLS analysis using the following Standard Operating Procedure (SOP) settings:
Material: protein
Solvent: PBS
Temperature: 25 °C
Equilibration time: 120 sec
Cuvette: ZMV 1012
Number of runs: 11
Measurement: 3
Note: Refer to equipment manual to set Standard Operating Procedure.
- Perform DLS analysis using Zetasizer μV and a 1-cm-path-length quartz cuvette (Hellma). Clean the cuvette with deionized water 3 times. Dry the cuvette. Place 4 μl of the BMV sample in the quartz cuvette.
- SDS PAGE
Recipes
- Inoculation buffer
0.01 M sodium phosphate
0.01 M magnesium chloride
Adjust pH to 6 - BMV extraction buffer
0.5 M sodium acetate
0.3 M boric acid
0.01 M magnesium chloride
Adjust pH to 4.5 - 10x L buffer (phosphate buffer)
0.02 M sodium phosphate
Adjust pH to 7.5
Acknowledgments
This work was supported by grant No. UMO-2012/06/A/ST4/00373 from the National Science Center (Poland). The above described BMV purification method was originally mentioned in our previous work: Strugala et al., 2017.
Competing interests: The authors declare no competing interests.
References
- Alejska, M., Malinowska, N., Urbanowicz, A. and Figlerowicz, M. (2005). Two types of non-homologous RNA recombination in brome mosaic virus. Acta Biochim Pol 52(4): 833-844.
- Arcangeli, C., Circelli, P., Donini, M., Aljabali, A. A., Benvenuto, E., Lomonossoff, G. P. and Marusic, C. (2014). Structure-based design and experimental engineering of a plant virus nanoparticle for the presentation of immunogenic epitopes and as a drug carrier. J Biomol Struct Dyn 32(4): 630-647.
- Bockstahler, L. E. and Kaesberg, P. (1962). The molecular weight and other biophysical properties of bromegrass mosaic virus. Biophys J 2(1): 1-9.
- Chen, C., Daniel, M. C., Quinkert, Z. T., De, M., Stein, B., Bowman, V. D., Chipman, P. R., Rotello, V. M., Kao, C. C. and Dragnea, B. (2006). Nanoparticle-templated assembly of viral protein cages. Nano Lett 6(4): 611-615.
- Dixit, S. K., Goicochea, N. L., Daniel, M. C., Murali, A., Bronstein, L., De, M., Stein, B., Rotello, V. M., Kao, C. C. and Dragnea, B. (2006). Quantum dot encapsulation in viral capsids. Nano Lett 6(9): 1993-1999.
- Dragnea, B., Chen, C., Kwak, E. S., Stein, B. and Kao, C. C. (2003). Gold nanoparticles as spectroscopic enhancers for in vitro studies on single viruses. J Am Chem Soc 125(21): 6374-6375.
- Figlerowicz, M. (2000). Role of RNA structure in non-homologous recombination between genomic molecules of brome mosaic virus. Nucleic Acids Res 28(8): 1714-1723.
- Guerrero, Y., Singh, S. P., Mai, T., Murali, R. K., Tanikella, L., Zahedi, A., Kundra, V. and Anvari, B. (2017). Optical characteristics and tumor imaging capabilities of near infrared dyes in free and nano-encapsulated formulations comprised of viral capsids. ACS Appl Mater Interfaces 9(23): 19601-19611.
- Huang, X., Stein, B. D., Cheng, H., Malyutin, A., Tsvetkova, I. B., Baxter, D. V., Remmes, N. B., Verchot, J., Kao, C., Bronstein, L. M. and Dragnea, B. (2011). Magnetic virus-like nanoparticles in N. benthamiana plants: a new paradigm for environmental and agronomic biotechnological research. ACS Nano 5(5): 4037-4045.
- Jung, B., Rao, A. L. and Anvari, B. (2011). Optical nano-constructs composed of genome-depleted brome mosaic virus doped with a near infrared chromophore for potential biomedical applications. ACS Nano 5(2): 1243-1252.
- Kao, C. C., Ni, P., Hema, M., Huang, X. and Dragnea, B. (2011). The coat protein leads the way: an update on basic and applied studies with the Brome mosaic virus coat protein. Mol Plant Pathol 12(4): 403-412.
- Ni, P., Vaughan, R. C., Tragesser, B., Hoover, H. and Kao, C. C. (2014). The plant host can affect the encapsidation of brome mosaic virus (BMV) RNA: BMV virions are surprisingly heterogeneous. J Mol Biol 426(5): 1061-1076.
- Ren, Y., Wong, S. M. and Lim, L. Y. (2007). Folic acid-conjugated protein cages of a plant virus: a novel delivery platform for doxorubicin. Bioconjug Chem 18(3): 836-843.
- Strugala, A., Krecisz, M., Rybka, J. D., Urbanowicz, A., Szpotkowski, K., Bierwagen, P., Figlerowicz, M., Kozak, M., Bottcher, C. and Giersig, M. (2017). Biophysical analysis of BMV virions purified using a novel method. J Chromatogr B Analyt Technol Biomed Life Sci 1068-1069: 157-163.
- Urbanowicz, A., Alejska, M., Formanowicz, P., Blazewicz, J., Figlerowicz, M. and Bujarski, J. J. (2005). Homologous crossovers among molecules of brome mosaic bromovirus RNA1 or RNA2 segments in vivo. J Virol 79(9): 5732-5742.
- Vaughan, R., Tragesser, B., Ni, P., Ma, X., Dragnea, B. and Kao, C. C. (2014). The tripartite virions of the brome mosaic virus have distinct physical properties that affect the timing of the infection process. J Virol 88(11): 6483-6491.
- Wierzchoslawski, R., Urbanowicz, A., Dzianott, A., Figlerowicz, M. and Bujarski, J. J. (2006). Characterization of a novel 5' subgenomic RNA3a derived from RNA3 of Brome mosaic bromovirus. J Virol 80(24): 12357-12366.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Strugała, A., Bierwagen, P., Rybka, J. D., Giersig, M., Figlerowicz, M. and Urbanowicz, A. (2018). BMV Propagation, Extraction and Purification Using Chromatographic Methods. Bio-protocol 8(14): e2935. DOI: 10.21769/BioProtoc.2935.
Category
Biochemistry > Protein > Isolation and purification
Microbiology > Microbe-host interactions > Virus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











