- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fluorophore-Based Mitochondrial Ca2+ Uptake Assay
Published: Vol 8, Iss 14, Jul 20, 2018 DOI: 10.21769/BioProtoc.2934 Views: 7575
Reviewed by: Gal HaimovichJosé M. DiasPia Giovannelli

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Automated Imaging Method for Quantification of Changes to the Endomembrane System in Mammalian Spheroid Models
Margaritha M. Mysior and Jeremy C. Simpson
Jun 5, 2025 1650 Views

Quantifying Intracellular Distributions of HaloTag-Labeled Proteins With SDS-PAGE and Epifluorescence Microscopy
Julia Shangguan and Ronald S. Rock
Jul 20, 2025 2501 Views

Fluorescence Lifetime-Based Separation of FAST-Labeled Cellular Compartment
Aidar R. Gilvanov [...] Yulia A. Bogdanova
Oct 5, 2025 1323 Views
Abstract
The physiological importance of mitochondrial calcium uptake, observed in processes such as ATP production, intracellular calcium signaling, and apoptosis, makes desirable a simple, straightforward way of investigating this event with unambiguous results. The following protocol uses a calcium-sensitive, membrane-impermeable fluorophore to monitor extra-mitochondrial calcium levels in the presence of permeabilized mammalian cells harboring activated mitochondria.
Keywords: MitochondrialBackground
Mitochondrial Calcium Uniporter (MCU)-mediated calcium flux is the primary way in which calcium enters the mitochondria. Mitochondrial calcium is important for several reasons, three of which are cited often in the literature. One, calcium in the mitochondria activates key dehydrogenases in the Krebs cycle which leads to increased ATP production. A more recent study indicates that mitochondrial calcium has a direct effect on both F1FO-ATPase and cytochrome chain activity (Glancy and Balaban, 2012), further enhancing its role as pivotal in cellular energy production. Two, because of the low, micromolar affinity of MCU and the large amount of cytosolic calcium mitochondria can sequester, MCU-mediated calcium uptake also plays a critical role in clearing transient increases in cytoplasmic calcium and in turn, shapes cellular signaling pathways that use calcium as a secondary messenger (Wheeler et al., 2012). Three, modulations of mitochondrial calcium play an important role in the regulation of apoptosis (Zoratti and Szabo, 1995). Steep increases in mitochondrial calcium levels initiate cell death by inducing the opening of the mitochondrial permeability transition pore in the inner membrane, an event that dissipates the inner mitochondrial membrane potential and releases cytochrome C, Diablo/Smac, and Caspase enzymes from the intermembrane space (Zoratti and Szabo, 1995; Pacher and Hajnoczky, 2001). While these phenomena have been well-characterized, the genetic identity of MCU has only recently been observed, and with that discovery has come the demand for a speedy and reliable way of observing mitochondrial calcium flux.
Our fluorophore-based mitochondrial calcium uptake assay is easy to set up and provides several advantages over other popular methods. HEK-293 cells with activated mitochondria are suspended in a recording buffer along with a membrane-impermeable calcium-sensitive fluorophore. The plasma membrane is permeated with a detergent while leaving the mitochondrial inner membrane in-tact, bringing the mitochondria in direct contact with the buffer. Following this, calcium is added to the cell-suspension and MCU-mediated calcium flux can be followed by observing the changing fluorescence of the fluorophore, which cannot follow calcium into the mitochondria. The highly specific MCU inhibitor, Ru360, is finally added to the cell-suspension to show that the observed change in fluorescence (i.e., calcium flux) is mediated by MCU. One of the most attractive features of the protocol is the speed of set-up and acquisition of flux data. Once cells are ready to harvest, calcium-flux data can be obtained in less than ten minutes. Another important quality of the protocol lies in its simplicity, specifically, in the straight-forward way in which the assay reports calcium flux and identifies MCU as the pathway. Implicating MCU as the sole calcium uptake pathway in this protocol is the observation that no calcium uptake of any kind is observed in cells lacking MCU. One drawback to the protocol is the inability of it to carefully quantify calcium flux, and for this, a calcium-45 uptake protocol is much preferable. In fact, limited quantification of mitochondrial calcium flux using this protocol is possible if one reports only the relative calcium flux, for example, by comparing two fluxes as a ratio of one over the other.
One of the opaquer yet more popular methods of observing MCU activity aims to follow the changes in mitochondrial calcium levels in intact cells whose mitochondria have been pre-loaded with a membrane-permeable calcium sensitive fluorophore. Because the plasma membranes of these cells are intact, intracellular calcium modulation depends on the release of calcium from the other major intracellular calcium sink, the endoplasmic reticulum (ER), which can be triggered by the addition of histamine to the extracellular buffer. Histamine achieves this by activating the phospholipase C/IP3 pathway, which results in the production of IP3 and concomitant activation of the IP3-receptor in the ER membrane, thereby releasing calcium stores from the ER into to cytoplasm. Because of the spatial proximity of the ER to the mitochondria, activation of the IP3 receptor transiently bathes mitochondria with a high dose of free calcium, which is in turn sequestered by the mitochondrial matrix. In this system, intra-mitochondrial calcium levels are monitored by observing changes in fluorescence of the pre-loaded, membrane-permeable, calcium sensitive probes which have presumably migrated to the mitochondrial matrix. Because MCU is the primary way through which calcium traverses the inner-mitochondrial membrane, it is taken for granted that the observed changes in fluorescence are due to the activation of MCU by these local increases in free calcium. It has even been suggested that the degree to which the fluorescence signal changes upon histamine stimulation is directly proportionate to the degree of MCU functionality. The complexity of this experimental design naturally raises doubts about what’s being inferred, namely, that the fluorescence changes upon histamine stimulation are a function of MCU functionality alone and not of, for example, the successful localization of the probe to mitochondria, or of the potential changes to any part of the phospholipase C/IP3 pathway, or of changes in proximity of the ER to mitochondria, or of the amount of calcium stored and/or released by the ER in various cell types under various experimental conditions, any of which may explain the observed fluoresce differences between the conditions tested and which may actually have little to do with MCU functionality. The experimental design described in detail below aims to reduce these sorts of ambiguities and to clearly report mitochondrial calcium flux mediated by MCU.
Materials and Reagents
- Pipette tips
- Cell culture dishes (Corning, catalog number: 430293 )
- Pasteur pipette
- 15 ml tube
- HEK 293 cells (Incubation: 37 °C, 5.0% CO2) (ATCC, catalog number: CRL-1573 )
- DMEM (high glucose, no glutamine) (store at 4 °C) (Thermo Fisher Scientific, GibcoTM, catalog number: 11960051 )
- Trypsin
- Premium fetal bovine serum (store at -20 °C) (Atlanta Biologicals, catalog number: S11150 )
- L-glutamine (store at -20 °C) (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081 )
- HEPES (store at RT) (Sigma-Aldrich, catalog number: H3375-1KG )
- Potassium chloride (KCl) (store at RT) (Fisher Scientific, catalog number: P217-500 )
- Potassium phosphate dibasic (K2HPO4) (store at RT) (Fisher Scientific, catalog number: P290-500 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (store at RT) (Fisher Scientific, catalog number: M33-500 )
- Potassium hydroxide (KOH) (store at RT) (for pH to 7.4) (Fisher Scientific, catalog number: P250-500 )
- Succinate (store at RT) (Sigma-Aldrich, catalog number: S3674-100G )
- Calcium Green 5N - hexapotassium salt (CG-5N) (Thermo Fisher Scientific, InvitrogenTM, catalog number: C3737 )
Note: Store at -20 °C as powder; 4 °C dissolved in ddH2O. Recommended: dissolve to a concentration of 0.5 μM for stock solution. - Digitonin (Sigma-Aldrich, catalog number: D141 )
Note: Store at -20 °C as powder. Dissolve in ddH2O immediately before use. Recommended: dissolve to a concentration of 30 mM for stock solution. - Ruthedium 360 (Ru360) (Santa Cruz Biotechnology, catalog number: sc-222265 )
Note: Store at -20 °C as powder; 4 °C dissolved in ddH2O. - Calcium Chloride Dihydrate (Fisher Scientific, catalog number: C79-500 )
Recommended: Dissolve in water to a concentration of 10 mM for stock solution. - Growth media (see Recipes)
- Wash buffer (WB) (see Recipes)
- Recording buffer (RB) (see Recipes)
Equipment
- Pipettes
- Incubator (Fisher Scientific, IsotempTM, catalog number: 13-255-26 )
- Centrifuge (Thermo Fisher Scientific, model: HeraeusTM LabofugeTM 400 , catalog number: 75008164)
- Quartz cuvette (Fisher Scientific, Fisherbrand, catalog number: 14-958-128 )
- Small stir bar (Hach, catalog number: 2095349 )
- Thermo Cell Holder with Stirrer (Hitatchi, model: 251-0148 )
- F-2500 Fluorescence spectrophotometer (Hitachi, model: 251-0090 )
Software
- IGOR Pro
- Microsoft Excel
Procedure
- Cell (HEK-293) preparation
- Carefully remove growth media from a confluent 10-cm dish (i.e., with a Pasteur pipette. See Note 3 for more on cell density). Add 10 ml WB (Wash Buffer) warmed to 37 °C to the plate with 10 ml pipet, pipetting up and down to gently lift cells from the bottom of the plate, and transfer WB-cell slurry to a 15 ml tube. These preparations are to be carried out on the bench at room temperature. If cells are strongly adherent and not easily dislodged by gentle up-and-down pipetting, add 0.7 ml of Trypsin (or just enough to evenly cover cells) warmed to 37 °C after washing cells once with 10 ml WB and let stand in a 37 °C incubator for 2-3 min. Remove cells from the incubator. Cells should now be easily displaced by gentle up-and-down pipetting with 10 ml WB. Transfer WB-cell slurry to a 15 ml tube.
- Gently pellet cells by centrifugation at room temperature at 1,000 x g for 5 min. Discard supernatant.
- Wash pellet by re-suspending cells in 10 ml WB, spin for 5 min at 1,000 x g, and discard supernatant.
- Resuspend cells in 2.5 ml recording buffer (RB), also warmed to 37 °C. Transfer 2.0 ml of this slurry to the quartz cuvette with a small stir bar. (The remaining 0.5 ml can be used separately to analyze protein content via Western Blot analysis. See Note 5 for more on Western Blots)
Note: Gently flick cuvette to remove bubbles before the start of the experiment, as these can create unwanted noise in the data.
- Carefully remove growth media from a confluent 10-cm dish (i.e., with a Pasteur pipette. See Note 3 for more on cell density). Add 10 ml WB (Wash Buffer) warmed to 37 °C to the plate with 10 ml pipet, pipetting up and down to gently lift cells from the bottom of the plate, and transfer WB-cell slurry to a 15 ml tube. These preparations are to be carried out on the bench at room temperature. If cells are strongly adherent and not easily dislodged by gentle up-and-down pipetting, add 0.7 ml of Trypsin (or just enough to evenly cover cells) warmed to 37 °C after washing cells once with 10 ml WB and let stand in a 37 °C incubator for 2-3 min. Remove cells from the incubator. Cells should now be easily displaced by gentle up-and-down pipetting with 10 ml WB. Transfer WB-cell slurry to a 15 ml tube.
- Flux experiment
- Place cuvette with RB-cell slurry into the cell-holder/stirrer affixed to the spectrophotometer. Turn on a stirrer and stir cells slowly (see Note 1).
- Set spectrophotometer to these parameters:
- Excitation wavelength: 506 nm
- Emission wavelength: 531 nm
- Em/Ex slit width: 2.5/2.5 nm
- Time scan
- 600 sec (or longer)
- 0 sec delay
- 600 sec (or longer)
- PMT Voltage: 700 Volts
- Response: 0.04 sec
- Data Mode: Fluorescence
- Excitation wavelength: 506 nm
- Start recording time scan. See Figure 1 below.
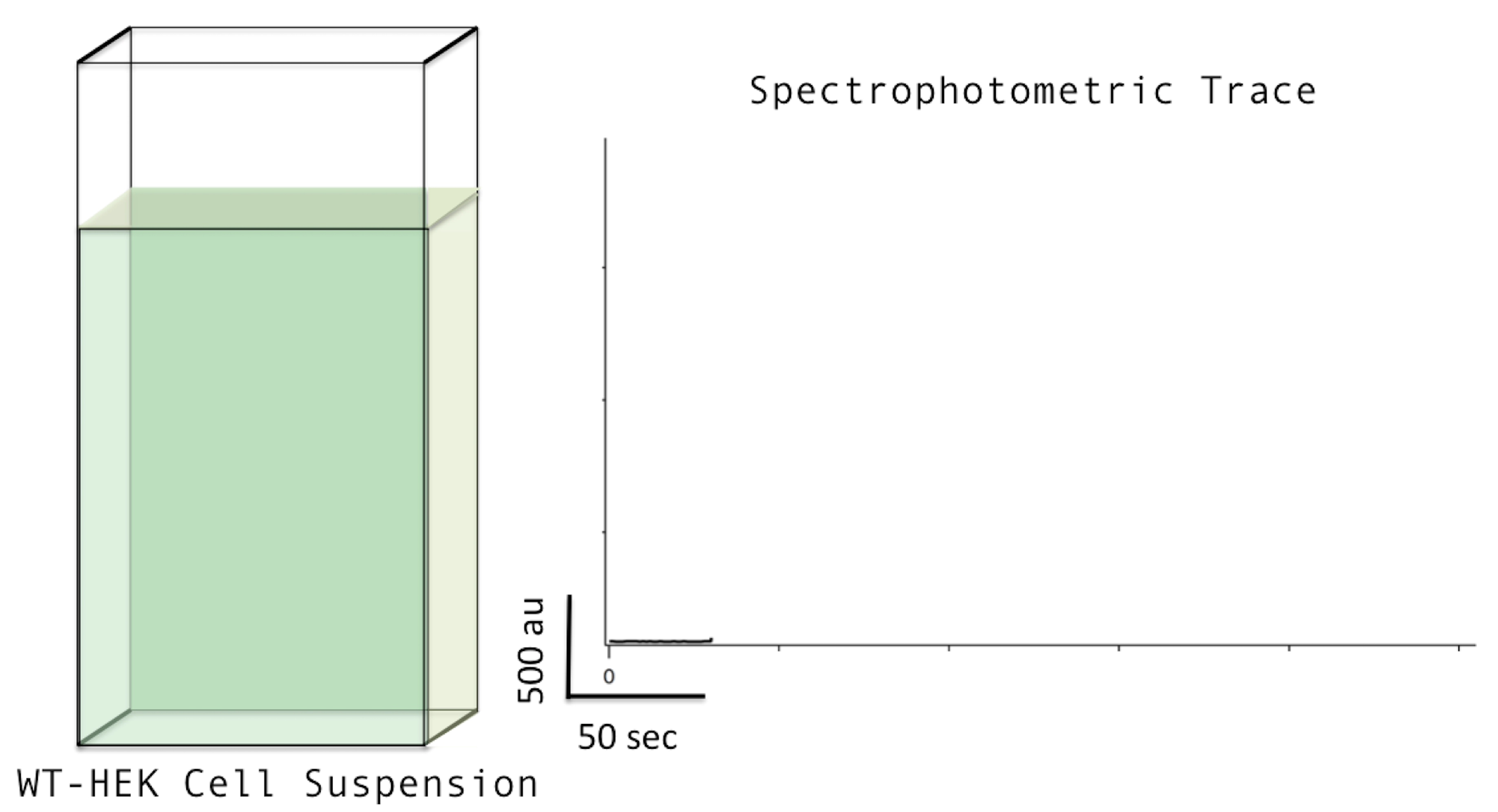
Figure 1. Starting the Recording. Left: cartoon of quartz cuvette with 2 ml recording buffer suspending wild-type (WT) HEK cells, loaded into the stirrer on the fluorescence spectrophotometer (small stir bar not shown). Right: spectrophotometric trace about 45 sec after starting the recording. - Add CG-5N to a final concentration of 0.25 nM (i.e., 1 μl of 0.5 μM stock). CG-5N is a membrane impermeable calcium-sensitive fluorophore (Kd = 14 μM). The fluorescence signal will go up with the addition of the fluorophore as trace amounts of calcium are present in the RB. See Figure 2 below.
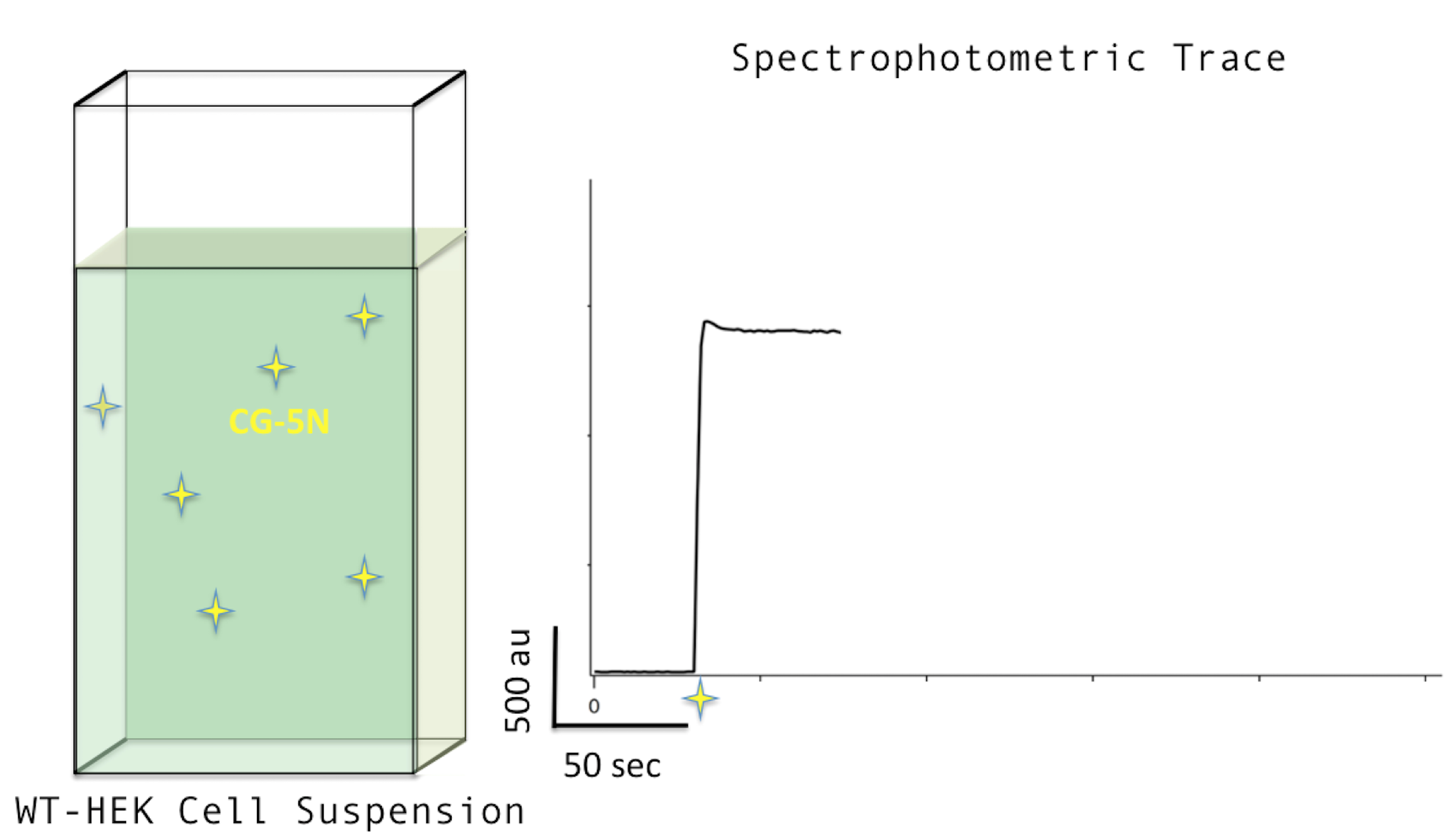
Figure 2. Adding CG-5N. Adding CG-5N to a concentration of 0.25 nM (left) will increase the fluorescence signal in the trace profile (right). - Add digitonin to a final concentration of 30 μM (i.e., 2 μl of 30 mM stock). Digitonin permeabilizes the plasma membrane by extracting cholesterol, leaving those intracellular membranes lacking cholesterol (e.g., the mitochondrial inner membrane) intact. This brings mitochondria in direct contact with the buffer solution and susceptible to influence by experimental reagents (the outer mitochondrial membrane is already in a steady-state with the cytoplasm with regards to small molecules like the reagents used in this protocol). CG-5N (membrane-impermeable) is afterward reporting ‘extra-mitochondrial’ calcium levels. A typical dip in fluorescence is almost always observed upon the addition of digitonin, as can be seen in Figure 3 below.
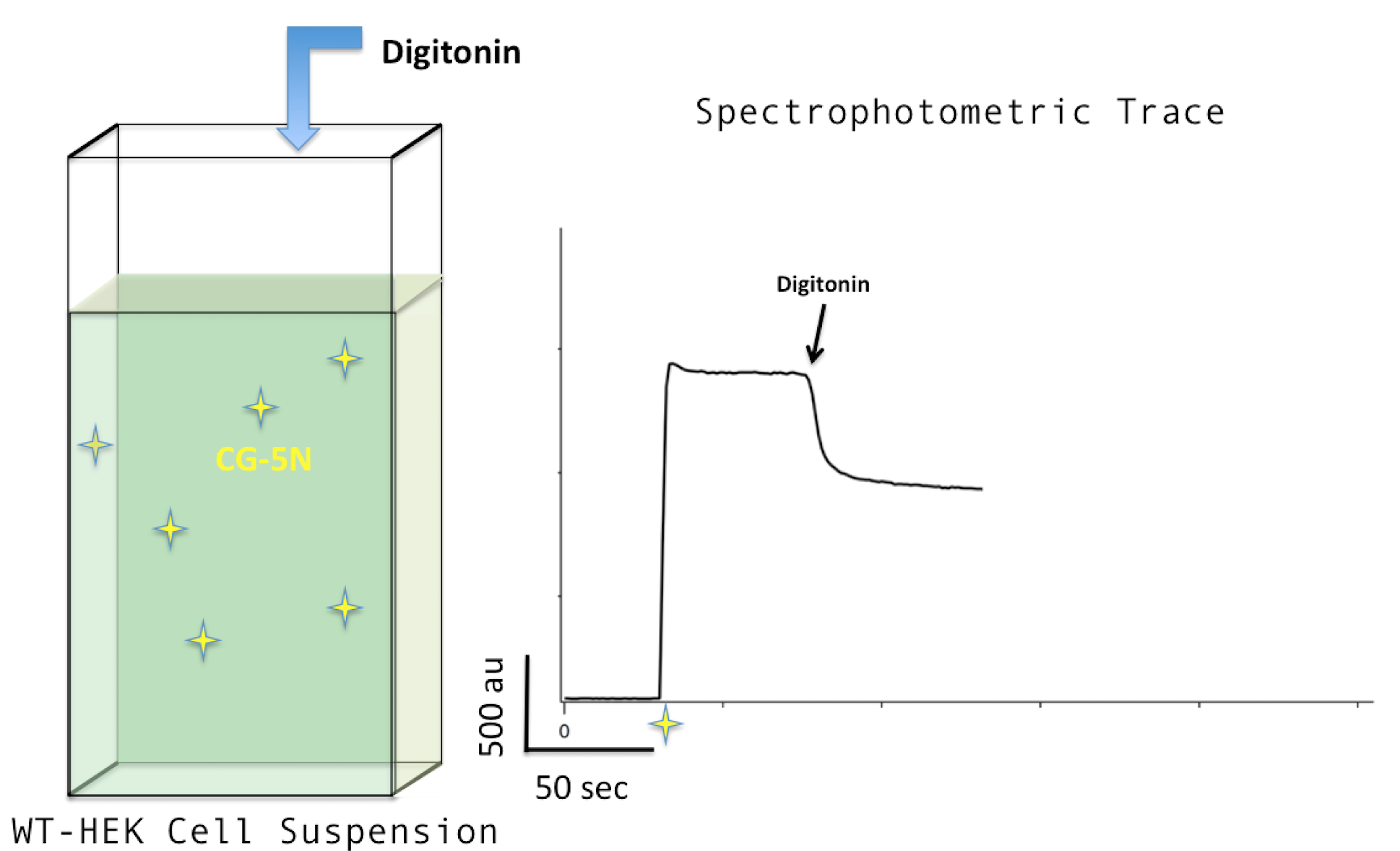
Figure 3. Adding digitonin. Adding digitonin to a concentration of 30 μM (left) will cause the spectrophotometric trace profile to drop (right). - Add CaCl2 to a final concentration of 10 μM (i.e., 2 μl of 10mM stock). The fluorescence signal will go up as calcium binds to CG-5N. If the cells are harboring activated mitochondria containing functional MCU (i.e., WT-HEK cells), a precipitous declination in fluorescence will immediately follow, signifying MCU-mediated calcium uptake (see Figure 4).
Note: If cells lack MCU, the fluorescence signal in the trace profile will increase but then will immediately flatten out, showing that the observed declination in the trace profile of WT cells after the addition of calcium is due to MCU-mediated calcium uptake AND NOT by other intra-cellular calcium uptake pathways such as SERCA (Sarco/Endoplasmic Calcium-ATPase) (Note 6).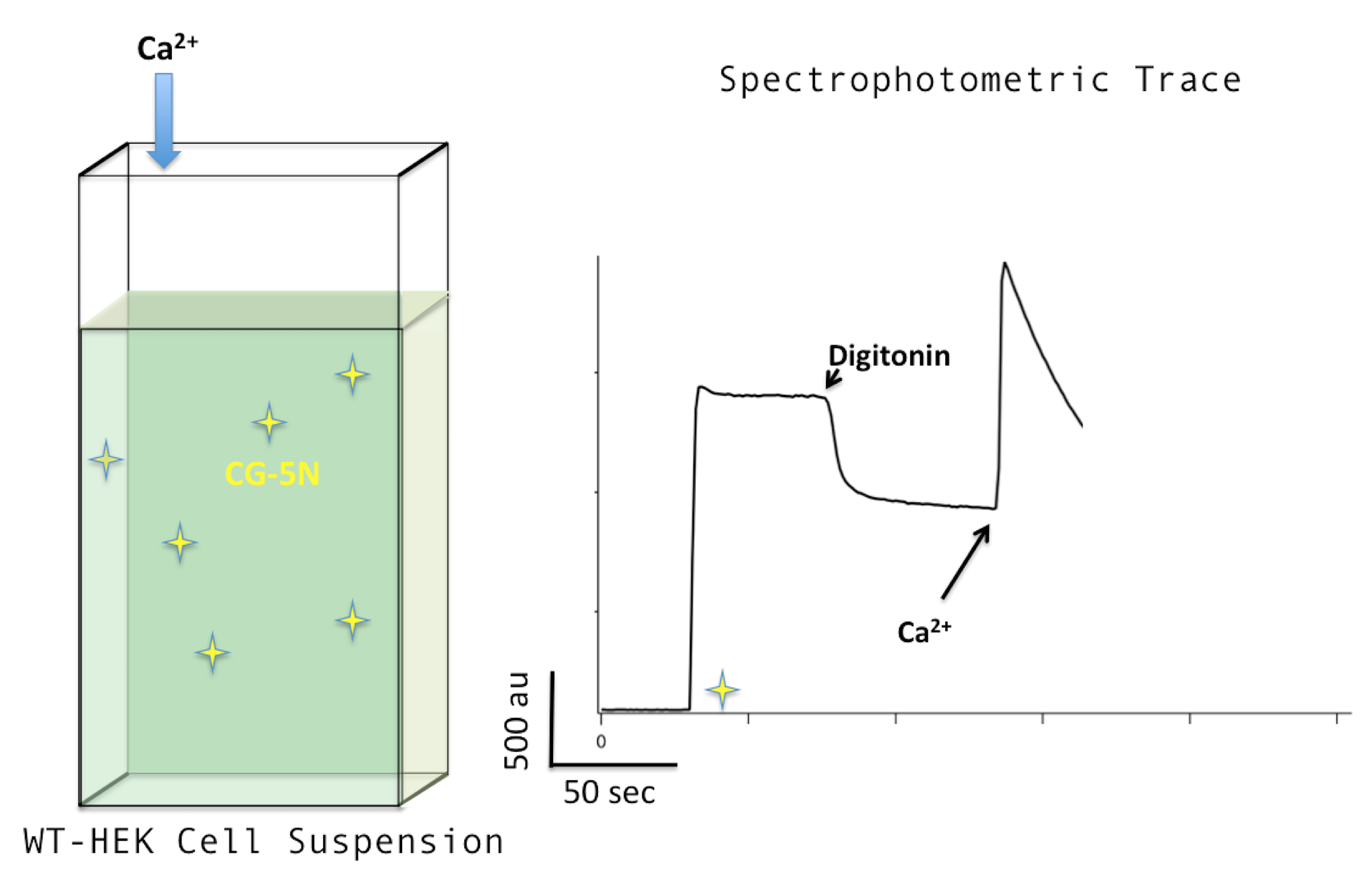
Figure 4. Adding calcium. Adding calcium to a final concentration of 10 μM (left) will produce a sharp increase in the trace profile (right). The following drop in fluorescence signal is indicative of MCU-mediated calcium flux. - After some time has passed, typically between 30 sec to 1 min, add Ru360 to a final concentration of 0.5 μM. Ru360 (i.e., 0.5 μl of 2 mM stock) is a potent inhibitor of MCU (Kd = 340 pM) and will cause the spectrophotometric trace to flatten as the steady-state exchange between the CG-5N-calcium-bound and CG-5N-calcium unbound species returns due to the lack of calcium mobility away from CG-5N. This step testifies to the dependence of MCU on the removal of calcium from the buffer solution observed in the previous step. See Figure 5 below; here, Ru360 is added to the cuvette after a second shot of 10 μM calcium.
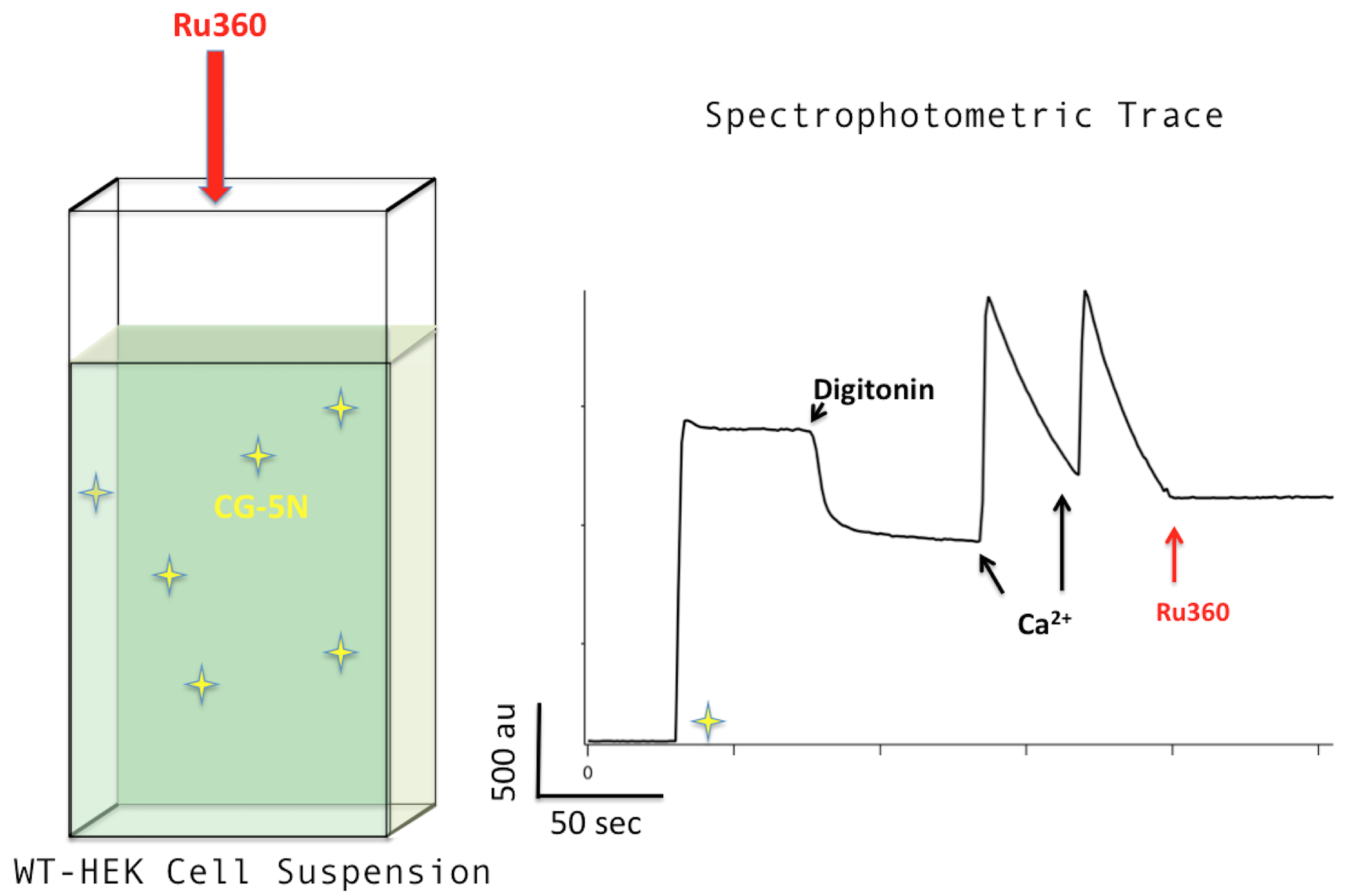
Figure 5. Ru360. Adding Ru360 to a final concentration of 0.5 μM (left) will cause the spectrophotometric trace profile to flatten (right).
- Place cuvette with RB-cell slurry into the cell-holder/stirrer affixed to the spectrophotometer. Turn on a stirrer and stir cells slowly (see Note 1).
Data analysis
- For reference data, please refer to Figure 4–figure supplement 2C, and Figure 4–figure supplement 3A in our paper ‘Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex’ by following the link provided below:
http://cdn.elifesciences.org/elife-articles/15545/figures-pdf/elife15545-figures.pdf?_ga=1.67863441.1487892113.1470405893 - Raw data from the spectrophotometric traces were uploaded into IGOR Pro and the slope of the a.u./time trace, starting immediately following the addition of calcium and for the following 10 sec, was calculated from each graph. The average of 3 slopes from cells expressing WT-hMCU and WT-hEMRE was calculated in Microsoft Excel along with the concomitant standard error. These values were used to normalize all following experimental data sets against.
Notes
- Stir bar speed
The speed of the stir bar can be an important but overlooked factor in determining the quality of the fluorescence trace profile. Spinning too fast can result in cell damage (cells will clump together in the cuvette), while spinning too slowly may limit the speed of reagent mixing within the cuvette, which can be seen in the spectrophotometric data primarily as enhanced noise and hyperbolic-like transitions from one steady-state to another upon the addition of a new reagent to the cuvette. In general, we’ve found that starting the stir bar in its slowest setting first and then slowly ramping up speed is the best way to find the optimal speed leading to intact cells and quick rates of reagent mixing. In our hands, this ideal speed is, qualitatively, on the slow end within the range of possible stir-bar speeds. - Digitonin
We have found that digitonin from Sigma-Aldrich works particularly well for this experiment. Also, make digitonin stock fresh before each experiment, as this reagent tends to crash out of solution between experiments (within an hour). - Cell density
While the number of HEK cells used in an experiment is completely up to the experimenter, we have found the ideal number to be 2.0 x 107, or 1 fully confluent 10 cm dish. - Cell types
We have performed this experiment exclusively with HEK 293 cells, and cannot say how it might work using other cell types. - Western Blot Analysis
Recording Buffer (RB) does not interfere Western Blot analysis. Simply spin the remaining 0.5 ml of cell slurry down, discard sup (RB), and lyse cells with ice cold lysis buffer (we found RIPA buffer works well for this). Lysing cells with about 50 μl RIPA buffer yields protein concentrations in a range appropriate for the comfortable loading of between 10 μg and 50 μg of protein in a 15 μl or 50 μl per well gel. A high-speed spin step at Step A4 is also required after lysis to pellet cell derbies and to keep the lysate from becoming ‘goopy’ and unmanageable during loading; keep the supernatant. Follow instructions for your lysis buffer of choice (add protease inhibitors, work on ice, etc.) and carefully quantify protein concentration after the high-speed spin with your method of choice. We always run a loading control gel on which we detect Actin (we load 10 μg protein for this blot). Other gels should be run on which to look for the protein of interest (typically MCU or one of its regulatory partners, but this obviously depends on the experiment and what’s desirable to detect). In general, expect to spend time optimizing western blots to see high-quality (high signal to noise) data. - A clear difference between the trace profiles of wild-type cells and MCU-knock out cells (cells in which MCU has been deleted from the genome) is that in the latter, the slope, after the addition of calcium, is nearly zero, while in the former, the slope is clearly negative. The profile’s negative slope after calcium addition is therefore indicative of MCU-mediated calcium uptake.
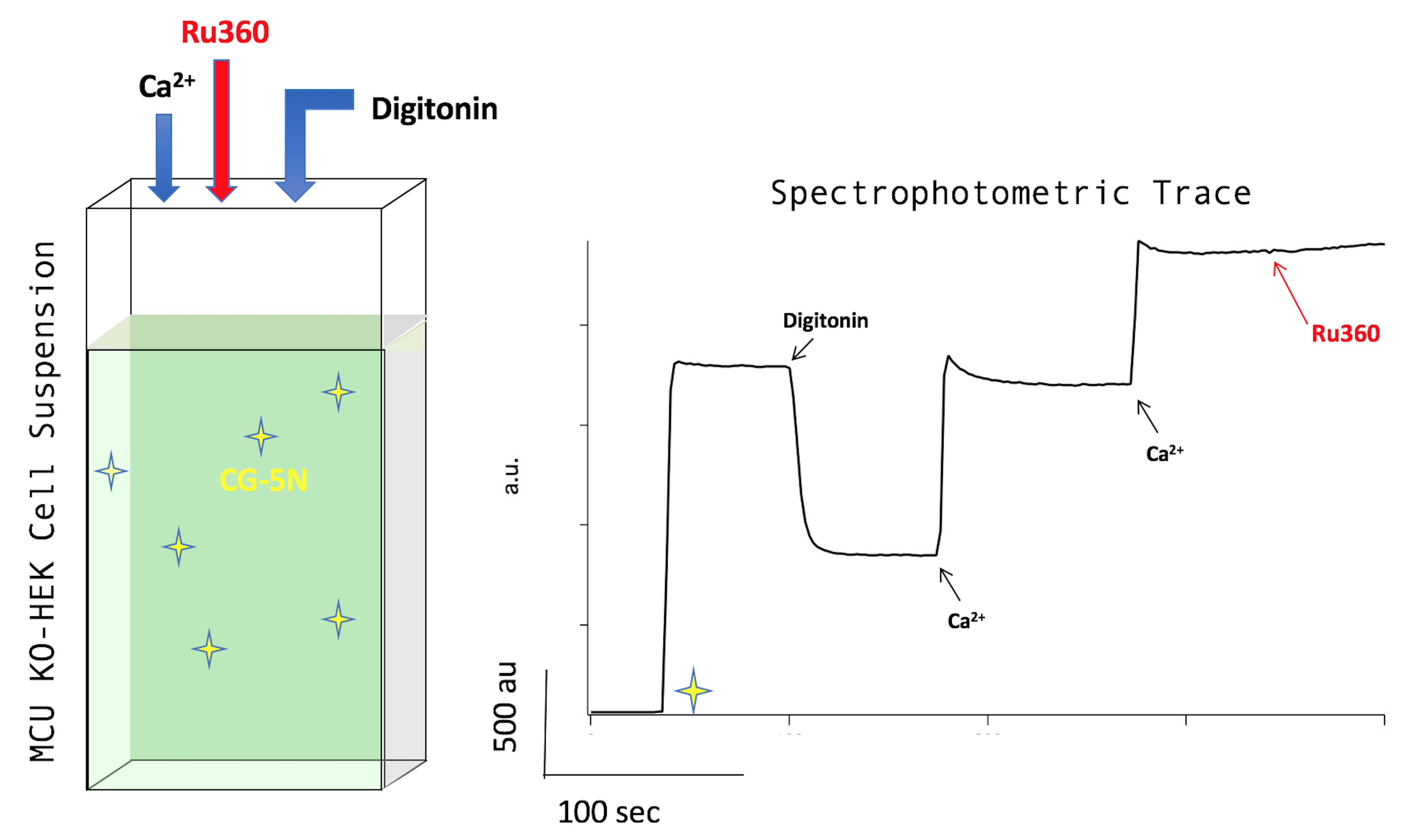
Figure 6. Spectrophotometric Profile of MCU-knockout Cells. Trace profile is flat after the addition of Ca2+ when MCU has been deleted from the genome.
Recipes
- Growth media
90% (v/v) DMEM
4.5 g/L D-glucose, L-glutamate, sodium pyruvate
10% (v/v) premium fetal bovine serum
2 mM L-glutamine
Store at 4 °C
Notes:- Prepare media under sterile conditions, i.e., in a laminar flow hood.
- Filter media through a 0.22 μm filter after preparation.
- Prepare media under sterile conditions, i.e., in a laminar flow hood.
- Wash buffer (WB)
20 mM HEPES
125 mM KCl
2 mM K2HPO4
1 mM MgCl2
Adjust pH to 7.4 with KOH
Store at 4 °C - Recording buffer (RB)
WB (see above)
5 mM succinate
Store at 4 °C
Acknowledgments
This work was carried out in Dr. Christopher Miller’s lab (HHMI; Brandeis University) and was supervised by Dr. Ming-Feng Tsai.
The above protocol is a classic protocol which has been used for decades in the field of mitochondrial calcium handling. Our thanks to those original thinkers who gave it life and to those who have refined it over the years. The author declares that there are no conflicts of interest or competing interests.
References
- Glancy, B. and Balaban, R. S. (2012). Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51(14): 2959-2973.
- Pacher, P. and Hajnoczky, G. (2001). Propagation of the apoptotic signal by mitochondrial waves. EMBO J 20(15): 4107-4121.
- Wheeler, D., Groth, R., Ma, H., Barrett, C., Owen, S., Safa, P., and Tsien, R. (2012). CaV1 and CaV2 channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell 149(5): 1112-1124.
- Zoratti, M. and Szabo, I. (1995). The mitochondrial permeability transition. Biochim Biophys Acta 1241(2): 139-176.
Article Information
Copyright
Phillips. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Phillips, C. (2018). Fluorophore-Based Mitochondrial Ca2+ Uptake Assay. Bio-protocol 8(14): e2934. DOI: 10.21769/BioProtoc.2934.
- Tsai, M. F., Phillips, C. B., Ranaghan, M., Tsai, C. W., Wu, Y., Willliams, C. and Miller, C. (2016). Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. Elife 5: e15545.
Category
Cell Biology > Cell imaging > Fluorescence
Cell Biology > Cell-based analysis > Ca2+ homeostasis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








