- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessment of Uptake and Biodistribution of Radiolabeled Cholesterol in Mice Using Gavaged Recombinant Triglyceride-rich Lipoprotein Particles (rTRL)
Published: Vol 8, Iss 13, Jul 5, 2018 DOI: 10.21769/BioProtoc.2916 Views: 6240
Reviewed by: Andrea PuharNelma Pértega-GomesTim Andrew Davies Smith

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualizing NBD-lipid Uptake in Mammalian Cells by Confocal Microscopy
Julia F. Baum [...] Thomas Günther Pomorski
Jul 5, 2023 2411 Views

Computational Analysis of Plasma Lipidomics from Mice Fed Standard Chow and Ketogenic Diet
Amy L. Seufert [...] Brooke A. Napier
Sep 20, 2023 2607 Views

Quantitative Determination of Cholesterol Hydroxylase Specificities by GC–MS/MS in Living Mammalian Cells
Hodaka Saito [...] Yoshio Yamauchi
Jan 20, 2024 2369 Views
Abstract
The here described method can be used to estimate the uptake of orally provided cholesterol in mice. Briefly, mice are gavaged with radiolabeled cholesterol and 4 h later, organ distribution of the radiolabel is determined by liquid scintillation counting. The method has been applied successfully to determine dietary cholesterol handling of mice housed at different ambient temperatures
Keywords: Lipid metabolismBackground
Cholesterol facilitates membrane fluidity and is a precursor for steroid hormones, bile acids and vitamin D. Animals are provided with cholesterol either from the diet or by de novo synthesis. Surplus of cholesterol may be harmful as its accumulation in blood vessel walls can cause atherosclerosis. It has been shown that the activation of brown adipose tissue (BAT) by cold reduces hypercholesterolemia (Berbee et al., 2015). It was, however, unclear whether BAT activity alters acute peripheral dietary cholesterol handling. To address this question, we orally administered mice with recombinant triglyceride-rich lipoprotein particles (rTRL) labeled with [4-14C]-Cholesterol and measured the organ distribution of the radiolabel 4 h after gavage. The biodistribution of radiolabeled cholesterol in vivo has been measured before (Szigeti et al., 1972; Townsend et al., 2001). However, in these studies, the radioactive cholesterol was either supplied by the diet or injected intravenously. Furthermore, other studies have assessed the fractional cholesterol absorption rate over a longer period of time (48 and 72 h) (Zilversmit and Hughes, 1974; Turley et al., 1994), As we were especially interested in the plasma clearance and the cholesterol uptake into the BAT after cold exposure, we chose to analyze the organs after a rather short period of time (4 h). Instead of applying an oil gavage, we used rTRL since the oil gavage resembles a rather unphysiologic condition (very high amount of lipids) but not a postprandial situation. Additionally, supplying the tracer with the diet is not suitable in cold-treated mice as they eat twice as much as when kept at warm ambient temperatures (30 °C).
Materials and Reagents
- Pipette tips
- Glass Vials 50 x 14 mm with plastic srew cap 5 ml (schuett-biotec, catalog number: 3563143 )
- Eppendorf Safe-Lock Tubes (Eppendorf, catalog number: 022363352 )
- 15 ml Tube PP (SARSTEDT, catalog number: 62.554.502 )
- Pico Prias Vial 6 ml (PerkinElmer, catalog number: 6000193 )
- Parafilm (IDL, catalog number: 2801310131 )
- Omnifix-F Syringes 1 ml (B. Braun Medical, catalog number: 9161406V )
- Animal Feeding needles (Fine Science Tools, catalog number: 18061-20 )
- 100 Sterican Hypodermic needle (B. Braun Medical, catalog number: 4657519 )
- C57BL/6 Mice (Charles River)
- Intralipid® 20% (Baxter, catalog number: 2B6064 )
- Methanol (Carl Roth, ROTISOLV®, catalog number: 7583.1 )
- Chloroform (Carl Roth, ROTISOLV®, catalog number: 4432.1 )
- NaCl 0.9% (B. Braun Medical, catalog number: 817403 )
- Cholesterol, [4-14C]-, 50 µCi (1.85 MBq) (PerkinElmer, catalog number: NEC018050UC )
- EDTA solution (Sigma-Aldrich, catalog number: E7889-100ml )
- Solvable (PerkinElmer, catalog number: 6NE9100 )
- Ketamin 10% (WDT, catalog number: 793-319 )
- Rompun® 2% (Bayer)
- Lipid solution (see Recipes)
- Anesthesia (see Recipes)
Equipment
- Aquasafe 500 Plus (Gardner Denver Medical, ZINSSER ANALYTIC, model: Aquasafe 500 Plus, catalog number: 1008500 )
- Balance (Sartorius, catalog number: BP 410 S )
- Pipette (Eppendorf, model: Research® plus, catalog number: 3123000063 ) with tips
- HeraeusTM FrescoTM 21 Microcentrifuge, Max. RCF: 21,100 x g (Thermo Fisher Scientific, model: HeraeusTM FrescoTM 21 , catalog number: 75002555)
- Nitrogen-Stream from Nitrogen bottle (Flow rate 1.5 L/min)
- Sonifier (Branson, model: Digital Sonifier® 450 )
- Sonifier Horn Double Step 1/8'' Microtip (Branson, catalog number: 101-063-212 )
- Vortexer (IKA, model: MS 1 )
- Climate chamber (Memmert, model: HPP750life )
- Beta-Counter (PerkinElmer, model: Tri-Carb® 2810TR )
Procedure
- Preparation of rTRL (see Figure 1 for a schematic overview)
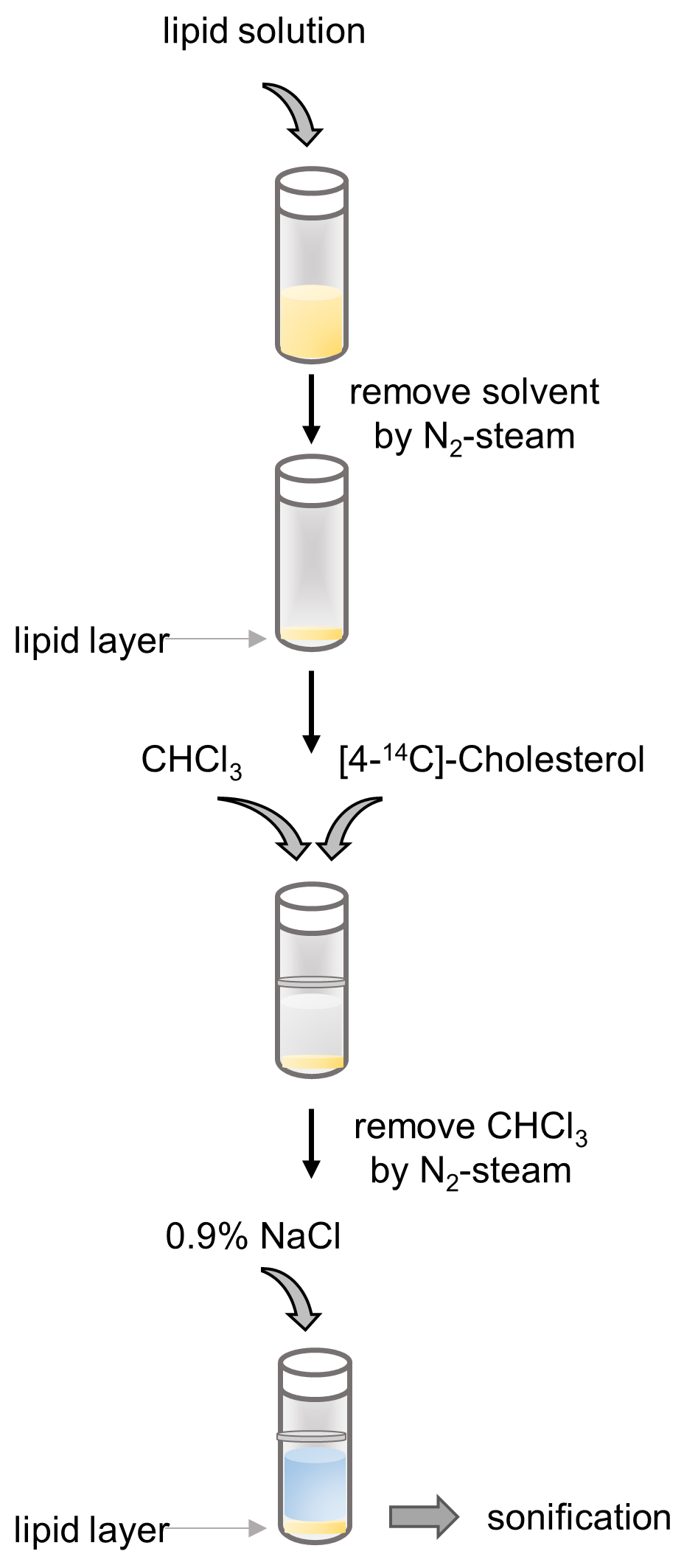
Figure 1. Schematic depiction of TRL Preparation
Note: All steps are performed at room temperature in a fume hood and in an isotope laboratory.- Add the lipid solution (see Recipes section below) to a glass vial (2 mg lipids per mouse) and make sure to prepare at least for 3 more mice than are required. Since we gavage 200 µl rTRL per mouse, minimum volume/vial would be for 4 mice (total final volume of 800 µl) and maximum volume/vial would be for 20 mice (total final volume of 4 ml).
- Remove solvents from lipid solution under an ambient flow of nitrogen (1.5 L/min) until you have a lipid layer at the bottom of the glass vial (Figure 2A).
- Add per mouse 50 µl of chloroform and 18.5 KBq of [4-14C]-Cholesterol to the glass vial.
- Remove the chloroform again by evaporating the lipid solution under a flow of nitrogen (1.5 L/min) until you again have a lipid layer at the bottom of the glass vial (Figure 1A).
- To form rTRL, add per mouse 200 µl of 0.9% NaCl solution to the lipid layer and seal vial with parafilm to avoid spilling during sonification (Figure 2B).
- Carefully place the vial below the sonifier tip. Make sure the tip does not touch the bottom (Figure 1B). Sonify three times for 3 min and 20 sec (20,000 Hz, 100 W) with a vigorous vortex step after the first sonification until the liquid has a milky appearance (Figure 2C).
- rTLR particles have to be prepared freshly and may not be stored overnight.
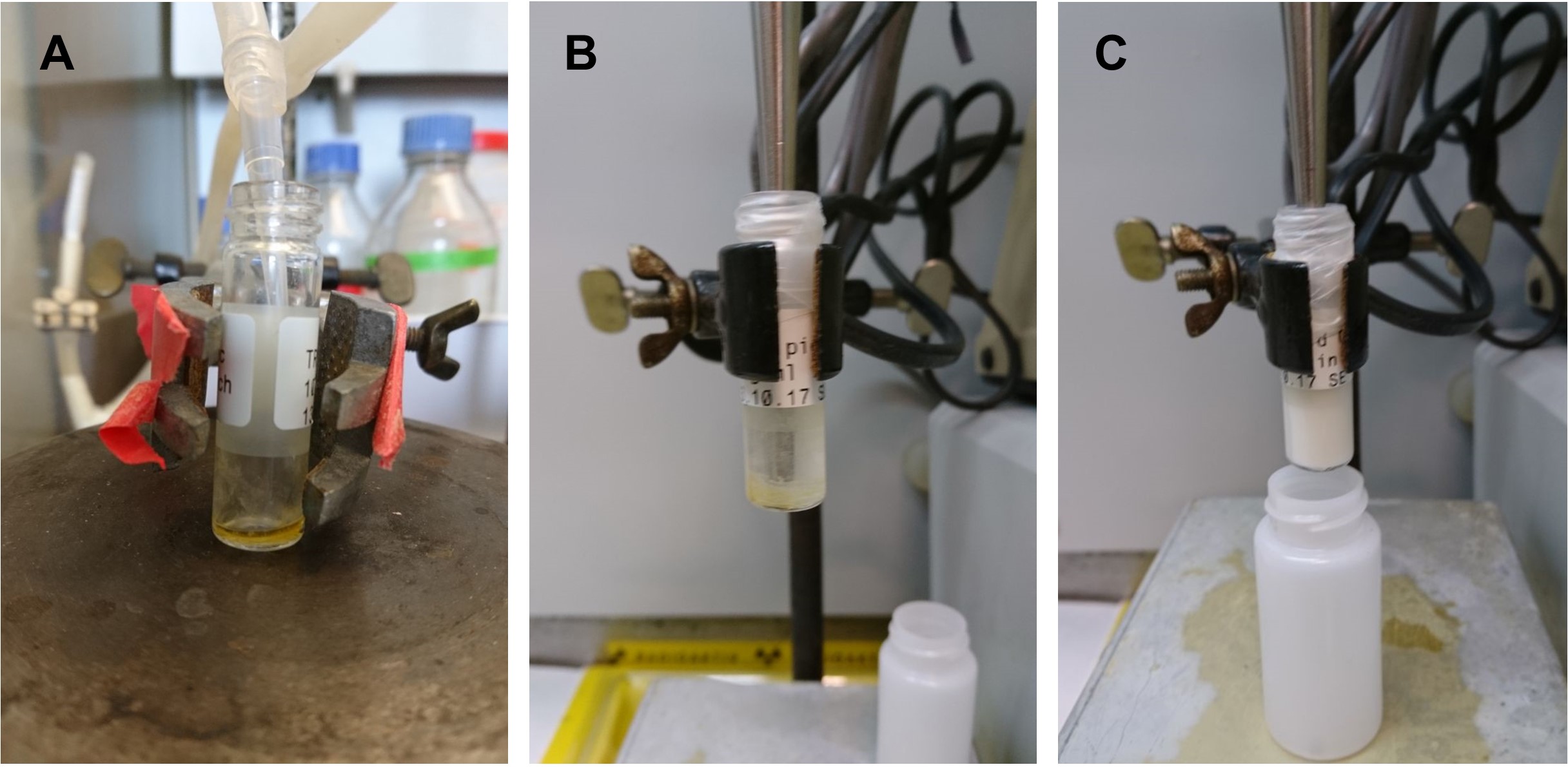
Figure 2. Preparation of the rTRL. A. Lipid solution was added to a glass vial and solvents were evaporated under a flow of nitrogen to leave an oily lipid layer on the bottom of the glass vial before and after addition of radioactivity. B and C. 0.9% NaCl solution was added to the lipid layer (B) and was sonified to form a milk-like rTRL emulsion (C).
- Add the lipid solution (see Recipes section below) to a glass vial (2 mg lipids per mouse) and make sure to prepare at least for 3 more mice than are required. Since we gavage 200 µl rTRL per mouse, minimum volume/vial would be for 4 mice (total final volume of 800 µl) and maximum volume/vial would be for 20 mice (total final volume of 4 ml).
- Cholesterol uptake assay
Note: All steps are performed at room temperature in an isotope laboratory.- Cholesterol uptake experiment is performed in mice previously housed at thermoneutrality (30 °C) or cold (6 °C) for 7 days in a humidity-controlled (55%) climate chamber.
- In the morning of the experiment fast the mice for 4 h. For this purpose, remove the food from the cages and refresh the bedding in the morning.
- After the 4 h fasting period, bring mice to the isotope laboratory (room temperature).
- For the gavage, fill rTRL solution into 1 ml syringes (equipped with an animal feeding needle as tip). Restrict mice by hand-grabbing and carefully place the feeding needle into their mouth and esophagus to empty 200 µl of rTRL solution (10 mg lipid/ml and 92.5 kBq/ml) directly into their stomach. Let the mice ingest for a further 4 h (water is provided at all times).
- For blood collection, prepare 1 ml syringes equipped with hypodermic needles and fill them with 5 µl EDTA.
- After four hours, anesthetize mice deeply by i.p. injection of 300 µl anesthesia (see Recipes section below).
- When mice are deeply anesthetized, open peritoneum, take 200 µl blood from the heart and then add the blood to an Eppendorf tube.
- Take organs of interest, note total weights of wet organs and store organ pieces (< 180 mg) each in a separate Eppendorf tube at 4 °C until further processing.
- Cholesterol uptake experiment is performed in mice previously housed at thermoneutrality (30 °C) or cold (6 °C) for 7 days in a humidity-controlled (55%) climate chamber.
- Liquid-Scintillation counting (see Figure 3 for a schematic overview)
Note: All steps are performed at room temperature in an isotope laboratory.- Prepare blood plasma by centrifugation of blood at 4 °C, 10,000 x g for 10 min. Take supernatant as plasma and store plasma at 4 °C until further processing.
- Dissolve organ pieces in 2 ml or 15 ml tubes with Solvable (100 µl per 10 mg organ) by shaking overnight at 60 °C. For scintillation counting, make sure to homogenize the solved organ thoroughly.
- Take 5 µl or 10 µl of rTLR solution or 10 µl of plasma and 400 μl of the organ solution and add 4 ml of Aquasafe 500 Plus in Pico Prias vials. Mix by shaking and let sit for an hour before liquid-scintillation counting (14C cpm mode).
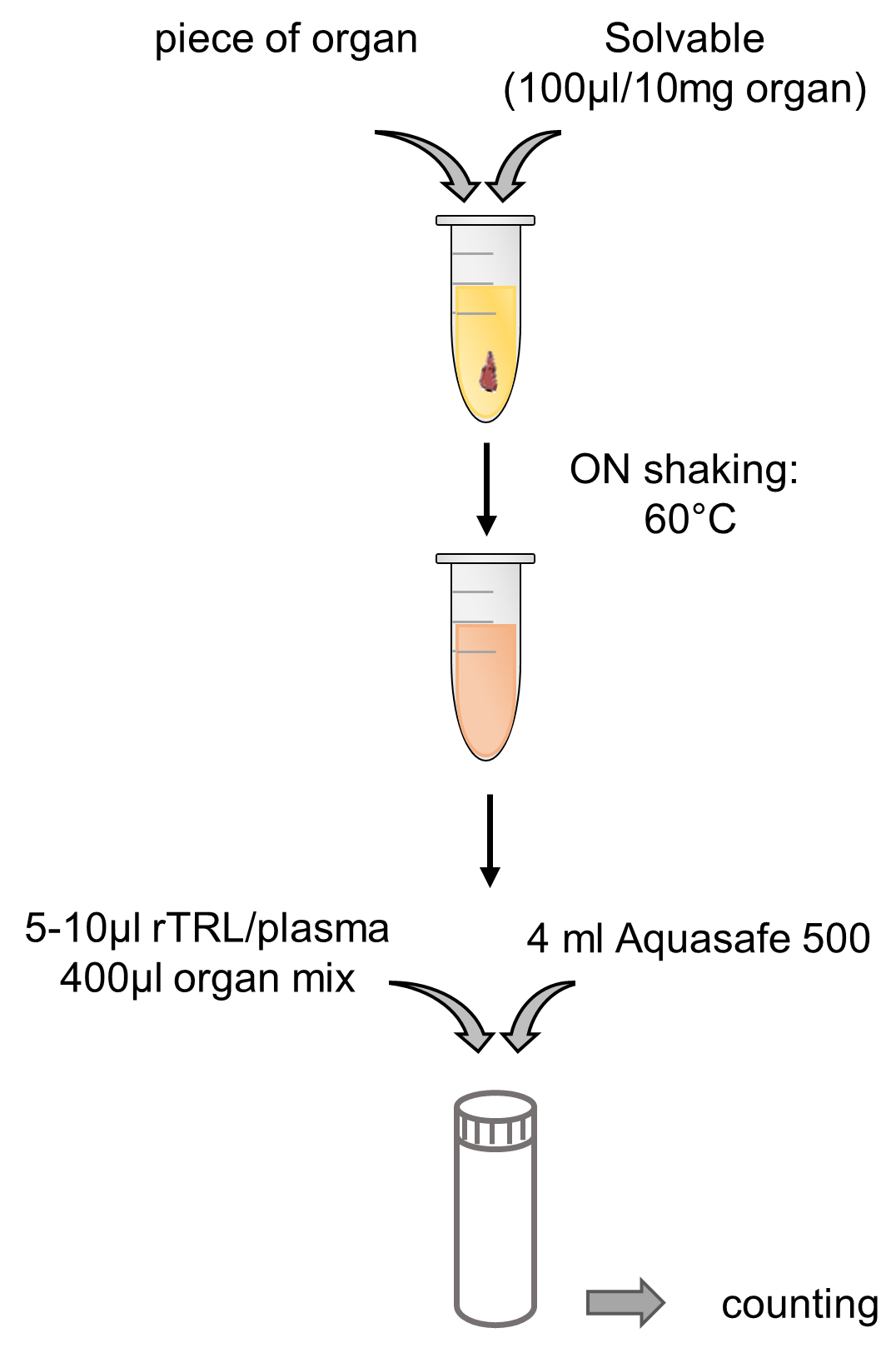
Figure 3. Schematic depiction of liquid-scintillation counting
- Prepare blood plasma by centrifugation of blood at 4 °C, 10,000 x g for 10 min. Take supernatant as plasma and store plasma at 4 °C until further processing.
Data analysis
Raw data from the counter was processed in Microsoft Excel. For every sample, counts per minute were measured twice and mean was calculated. Exemplary calculation for the rTRL solution is depicted below. As shown in Figure 4A, and as expected, the number of counts in each duplicate was roughly equal. Further, it was expected that counting 10 µl of rTRL solution would roughly result in twice as many counts detected in 5 µl TRL solution which is shown in Figure 4A. Additionally, calculated counts per µl were equal (Figure 4B).
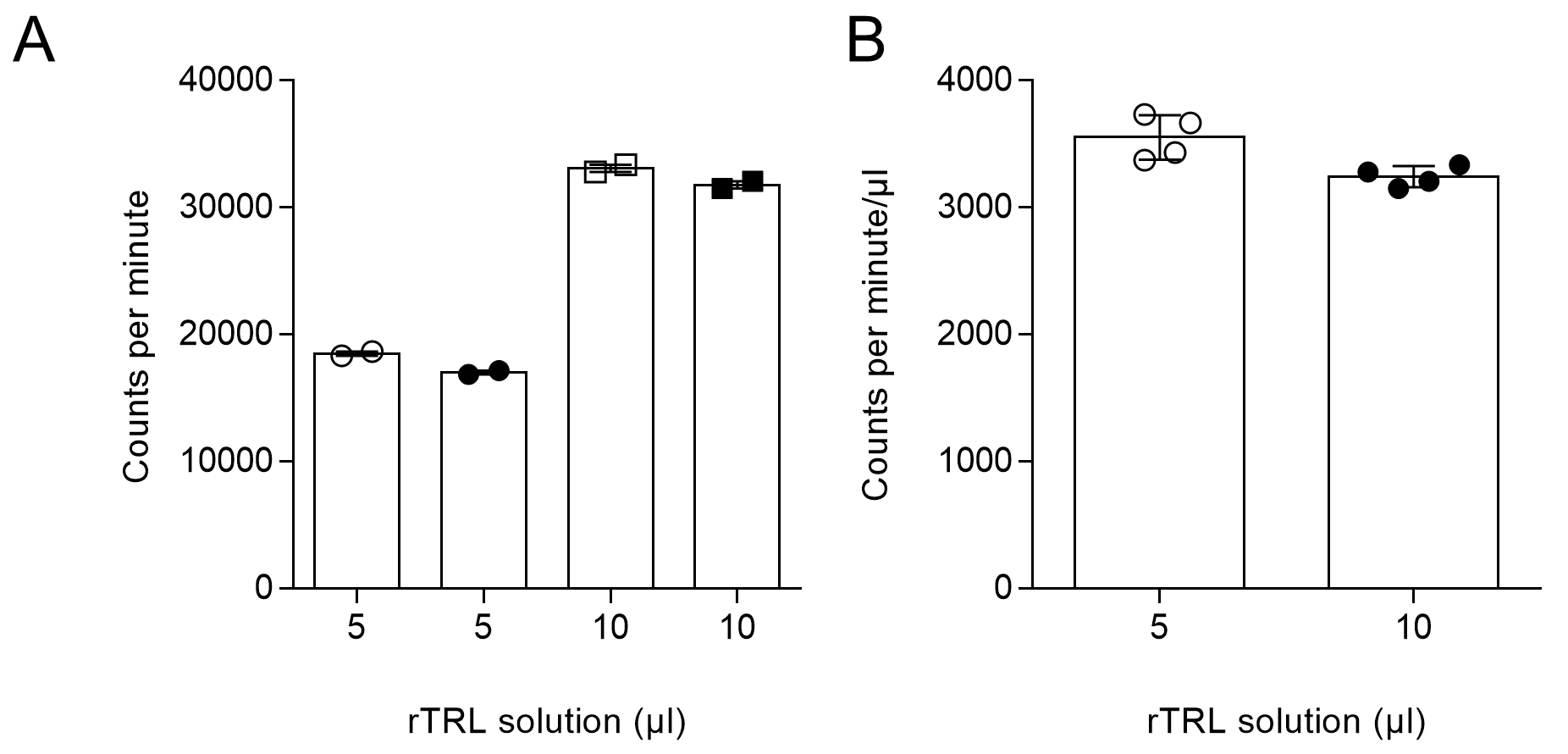
Figure 4. Counts in rTRL solution. A. Five or ten microliters of rTRL solution were counted in duplicates and counts per minute were detected. B. Five or ten microliters of rTRL solution were counted in and counts per minute/µl were calculated.
- Cholesterol uptake for every organ was then calculated per mg organ (counts divided by mass of organ piece), per total organ (counts per mg x total organ weight) or as percentage of given dose (counts per total organ/counts of given rTRL).
- To assess the uptake of cholesterol into different organs of one week cold exposed mice compared to warm-housed control mice, we used 7 biological replicates (7 mice per group) (Worthmann et al., 2017). As depicted in Figure 1j/k in Worthmann et al. 2017, cholesterol uptake into organs was presented as total organ radioactivity and % of given dose. The warm housed control group was set as 100%. Statistically significant differences in organ radioactivity were then calculated by unpaired two-tailed Student’s t-test. Cold exposure resulted in an increased cholesterol uptake into the brown adipose tissues and the liver (Worthmann et al., 2017).
Recipes
- Lipid solution
Notes: Make sure to prepare the lipid solution at least for 3 more mice than required.- Extract per mouse 2 mg lipids either from Intralipid 20% or from human TRL particles of a type 1 hyperlipidemia patient (patient described before in Bruns et al. [2009]) in a 2 ml Eppendorf Tube (make sure it is originally from Eppendorf, since it is resistant to solvents)
- Add per part lipid solution 4 parts chloroform/methanol (2:1 v/v) and vigorously shake it
- Then add 0.5 parts of chloroform/water (1:1 v/v) to the solution and centrifuge at 18,000 x g and 4 °C for 15 min to allow phase separation
- Carefully take lower phase with a normal Eppendorf pipette (volume 100-1,000 µl)
- The lipid solution can be stored in a glass vial at -80 °C for up to one year
- Extract per mouse 2 mg lipids either from Intralipid 20% or from human TRL particles of a type 1 hyperlipidemia patient (patient described before in Bruns et al. [2009]) in a 2 ml Eppendorf Tube (make sure it is originally from Eppendorf, since it is resistant to solvents)
- Anesthesia
Mix 2.3 ml Ketamin, 1 ml Rompun and 6.7 ml 0.9% NaCl in a 15 ml Tube
Acknowledgments
This protocol is adapted from Bruns et al. (2009) and Worthmann et al. (2017). This work was supported by grants funded by the Deutsche Forschungsgemeinschaft (SFB841; Liver inflammation: Infection, immune regulation and consequences). The authors have no conflicts of interest to declare.
References
- Berbee, J. F., Boon, M. R., Khedoe, P. P., Bartelt, A., Schlein, C., Worthmann, A., Kooijman, S., Hoeke, G., Mol, I. M., John, C., Jung, C., Vazirpanah, N., Brouwers, L. P., Gordts, P. L., Esko, J. D., Hiemstra, P. S., Havekes, L. M., Scheja, L., Heeren, J. and Rensen, P. C. (2015). Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 6: 6356.
- Bruns, O. T., Ittrich, H., Peldschus, K., Kaul, M. G., Tromsdorf, U. I., Lauterwasser, J., Nikolic, M. S., Mollwitz, B., Merkel, M., Bigall, N. C., Sapra, S., Reimer, R., Hohenberg, H., Weller, H., Eychmuller, A., Adam, G., Beisiegel, U. and Heeren, J. (2009). Real-time magnetic resonance imaging and quantification of lipoprotein metabolism in vivo using nanocrystals. Nat Nanotechnol 4(3): 193-201.
- Szigeti, M., Monnjer, G., Jacotot, B., Navarro, M. N. and Robert, L. (1972). Distribution of ingested 14C-cholesterol in the macromolecular fractions of rat connective tissues. Connective Tissue Research 1:145-152.
- Townsend, R. W., Zutshi, A. and Bekersky, I. (2001). Biodistribution of 4-[14C]cholesterol-AmBisome following a single intravenous administration to rats. Drug Metab Dispos 29(5): 681-685.
- Turley, S. D., Herndon, M. W. and Dietschy, J. M. (1994). Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J Lipid Res 35(2): 328-339.
- Worthmann, A., John, C., Ruhlemann, M. C., Baguhl, M., Heinsen, F. A., Schaltenberg, N., Heine, M., Schlein, C., Evangelakos, I., Mineo, C., Fischer, M., Dandri, M., Kremoser, C., Scheja, L., Franke, A., Shaul, P. W. and Heeren, J. (2017). Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med 23(7): 839-849.
- Zilversmit, D. B. and Hughes, L. B. (1974). Validation of a dual-isotope plasma ratio method for measurement of cholesterol absorption in rats. J Lipid Res 15(5): 465-473.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Worthmann, A., John, C. and Heeren, J. (2018). Assessment of Uptake and Biodistribution of Radiolabeled Cholesterol in Mice Using Gavaged Recombinant Triglyceride-rich Lipoprotein Particles (rTRL). Bio-protocol 8(13): e2916. DOI: 10.21769/BioProtoc.2916.
Category
Biochemistry > Lipid > Lipid transport
Biochemistry > Lipid > Lipid measurement
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










