- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Modified Approach for Axenic Cultivation of Spores of Fern Adiantum capillus-veneris L. with High Germination Rate
Published: Vol 8, Iss 13, Jul 5, 2018 DOI: 10.21769/BioProtoc.2914 Views: 5430
Reviewed by: Shweta PanchalShankar PantAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Modified Pseudo-Schiff Propidium Iodide for Staining the Shoot Apical Meristem in Arabidopsis
Ruiqi Li [...] Ligeng Ma
May 5, 2023 2269 Views

A Novel Imaging Protocol for Investigating Arabidopsis thaliana Siliques and Seeds Using X-rays
Brylie A. Ritchie [...] Ansul Lokdarshi
Oct 5, 2023 2182 Views

Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Pradeep Chand Deo
Oct 20, 2025 1426 Views
Abstract
Spores are the primary way of spread and reproduction for ferns, a clade of seed-free vascular plants. However, no detailed protocol for ferns spore cultivation has been reported yet. Here we provide a modified approach for axenic cultivation of fern Adiantum capillus-veneris L., based on Cao’s and Li’s method (Cao, et al., 2010; Li, et al., 2013).
Our approach can be briefly divided into four steps: 1) collect spores; 2) sterilize the spores with 5% sodium hypochlorite solution and wash twice; 3) incubate the spores in liquid Knop’s medium in the dark for five days; 4) cultivate the spores on Knop's plate medium. To increase the germination rate, we constrain the sterilization time under 25 min and add dark treatment step after spore sterilization. After these modifications, the germination rate raises from 2% to 25%.
Background
In the phylogenetic tree of plants, ferns are a critical clade of land plants, as they are the sister lineage of seed plants. Ferns are characteristic by their unique life cycle. Compared with seed plants, the sporophyte of ferns bear spores for spreading instead of seeds. Under favorable conditions, spores germinate to form gametophytes, in which cells on certain areas (rib) specialize into reproductive organs to produce gametes. After water-dependent fertilization, the newborn sporophyte emerge and an entire life cycle of fern completes (Li et al., 2013). Hence, to study the sexual reproduction processes and life history of ferns, spore cultivation under experimental conditions is necessary.
However, protocols available for ferns spores cultivation are insufficient. Here, we choose Adiantum capillus-veneris L. as experimental material to optimize the spore cultivation protocol, as Adiantum is a representative fern with isospory, and the cultivation system of Adiantum under lab condition has been developed by Li et al. (2013).
This protocol is also instructive for spore cultivation in other members of homosporous ferns.
Materials and Reagents
- Pipette tips (Corning, AXYGEN®, catalog number: T-1000-B )
- Sieve (with pore size no less than 0.20 mm, any brand)
- Sterile 1.5 ml microcentrifuge tubes (Eppendorf®)
- Petri dishes (NEST Biotechnology, catalog number: 715001 )
- Laboratory film (Parafilm® M)
- Adiantum spores
- Sterilized water
- NaClO (ALADDIN, catalog number: S101636-5kg )
- Ca(NO3)2 (ALADDIN, catalog number: C100076-100g )
- KNO3 (ALADDIN, catalog number: P111635-500g )
- MgSO4 (Sigma-Aldrich, VETECTM, catalog number: V900066-500G )
- KH2PO4 (Sigma-Aldrich, VETECTM, catalog number: V900041-500G )
- FeCl3·6H2O (Sigma-Aldrich, catalog number: 31232-250G )
- Gellan Gum (GelzanTMCM, PhytoTechnology Laboratories, catalog number: G3251 )
- Knop’s medium liquid (see Recipes)
- Knop’s medium solid (see Recipes)
Equipment
- 1 L flask (any brand)
- Sterile tweezers (any brand)
- Pipettes (Eppendorf®)
- Autoclave (Panasonic, model: MLS-3781L-PC )
- Super clean bench (Esco Micro, model: SVE-4A1 )
- Centrifuge (Thermo Fisher Scientific, model: HeraeusTM PicoTM 21 )
- Constant temperature and illumination intensity incubator (Beijing Luxi, model: GZX-400PY )
- Stereoscopic microscope (Leica Microsystems, model: Leica DFC450 C )
Procedure
- Collect the pinnae with mature sori. To ensure the pinnae are dried out, wrap them in a dry paper for 1-2 days. Place the dried out pinnae on a sieve and stir them to get dark brown sporangia and yellow spores.
Note: Sporangia and spores can be stored at 4 °C. For a high germination rate, immediate cultivation is recommended. - Thoroughly clean a sterile bench with 15 min UV radiation.
- Pour 2 mg of sporangia into a sterile 1.5 ml centrifuge tube, and then add 1,000 μl of 5% sodium hypochlorite solution into the centrifuge tube. Shake the centrifuge tube to ensure that sporangia are completely suspended. Lay the tube horizontally for 2 min (Video 1). Then add 500 μl of 5% sodium hypochlorite solution into a new centrifuge tube.Video 1. Sterilization of sporangia and release of spores from sporangia
- Pipette up and down to suspend the sporangia. After blowing out liquid, dark brown sporangia settle first, while light yellow spores settle slowly. At the time that sporangia settled while spores are still suspended, carefully transfer spores to the new disinfectant-containing tube (Video 2).
Note: Be careful not to draw up any sporangia, which increase the pollution risk.Video 2. Separation of spores from sporangia - Shake the centrifuge tube to ensure that spores are completely suspended. Lay the tube horizontally for 2 min. Then centrifuge for 2 min with 12,000 x g at room temperature.
Note: To ensure high germination rate of, we finished the whole sodium hypochlorite sterilize process within 25 min (Figure 1).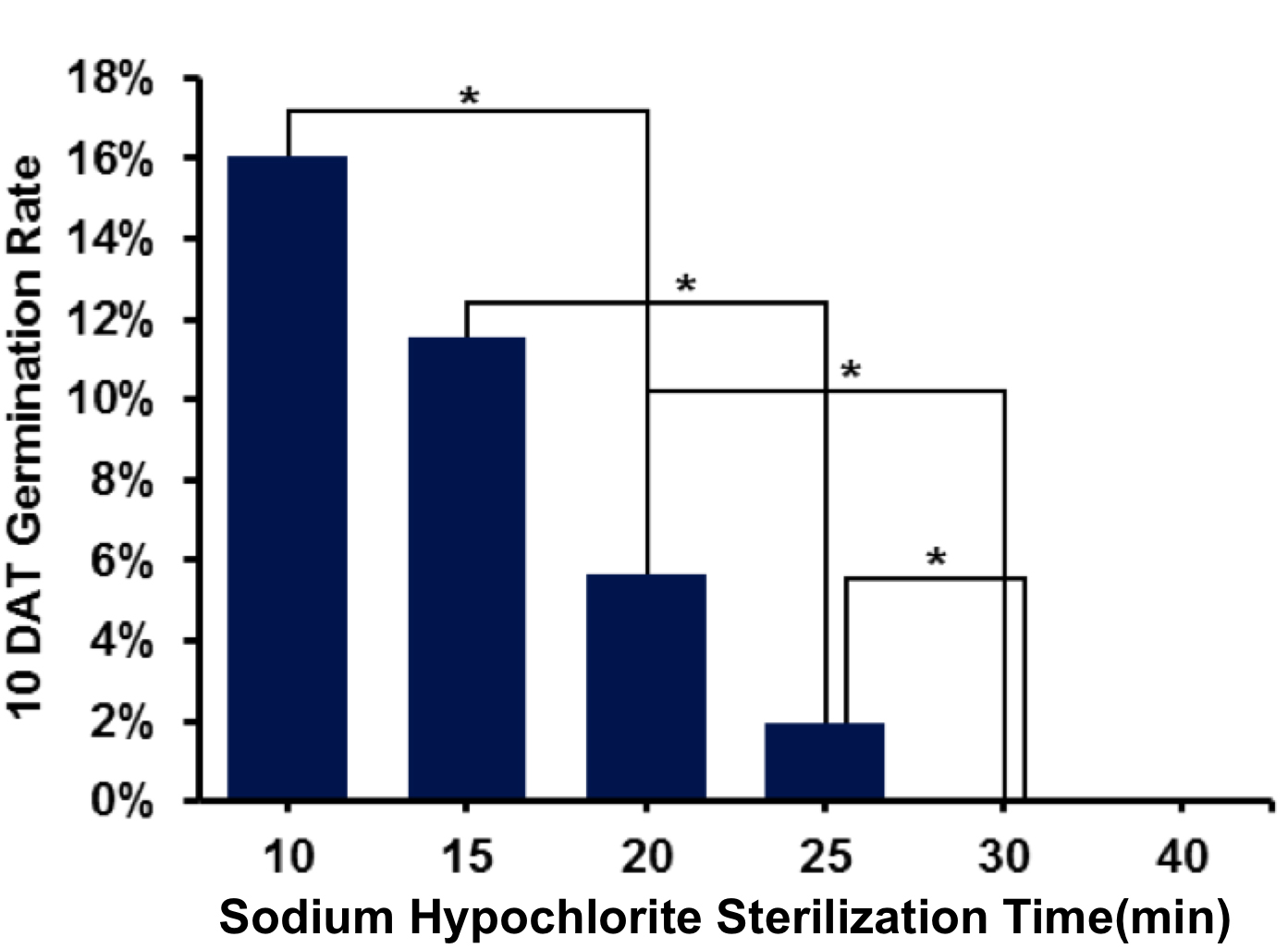
Figure 1. Germination rate decreased with the increasing of sterilization time. * stands for significant difference, P < 0.05. - Decant the supernatant from the centrifuge tube with a pipette. Wash spores with 1,000 μl sterile water. Shake the centrifuge tube to ensure that spores are completely suspended. Then centrifuge for 2 min at 12,000 x g at room temperature.
- Repeat Step 6.
- Decant the supernatant from the centrifuge tube with a pipette. Add 1,000 μl of liquid Knop's medium to the centrifuge tube (see Recipes). Shake the centrifuge tube to ensure that spores are completely suspended. Then wrap the tube with laboratory film.
- Place the spores in the dark at 25 °C for 5 days (Figure 2). After dark treatment, place spores under red light at 25 °C to induce germination. Alternatively, spores can be taken out of the dark and inoculated into solid Knop's plate, then placed under red light at 25 °C to induce germination (see Recipes).
Notes:- To increase the germination rate, dark treatment is required. We optimized the time of dark treatment from 0 to 5 days, and we found that germination rate is promoted as the dark treatment days increase (Figure 2).
- The wavelength of red light should be within range of red light, but not far-red light. Far-red light treatment will repress spores germination.
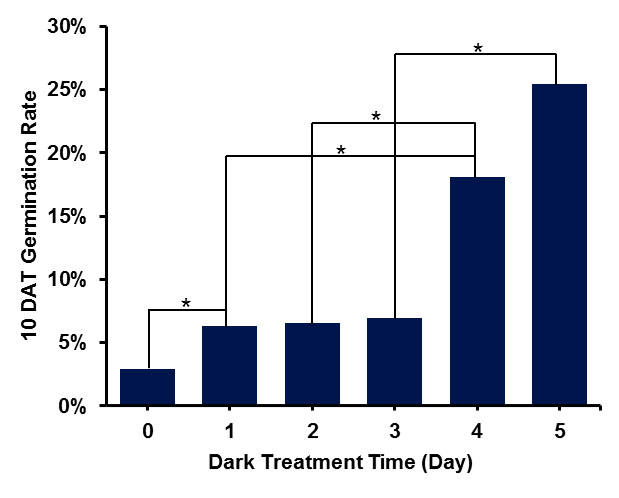
Figure 2. Dark treatment will increase spore germination rate. * stands for significant difference, P < 0.05. The germination can reach 25% when the spores were placed in the dark for 5 days. - Observe spores under a stereomicroscope. After 2 days of red light treatment, spores begin to germinate. They become large, round and with high density as the first mitosis begin. (Figure 3A). After 4-5 days of red light treatment, spores grow rhizoids (Figure 3B). Count the spores with rhizoids to calculate germination rate.
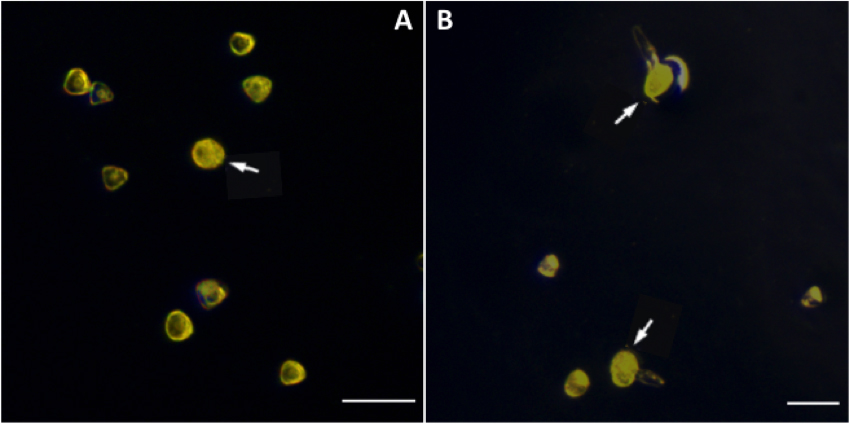
Figure 3. Development of Adiantum spores. A. Adiantum spores begin to germinate after 2 days of red light treatment (arrow line). B. Adiantum spores begin to grow rhizoids after 5 days of red light treatment (arrow line). Scale bars = 100 μm.
Data analysis
For experimental design, to compare two or more data sets, we use a minimum of three replicates for each set. For each replicate, we analyze a minimum of 12 photomicrographs, to ensure that each photomicrograph contains 30-40 spores, and the total number of spores counted reaches 400.
For data analysis, to calculate the germination rate of Adiantum spores, we take random photomicrographs of each solid Knop’s plate on which spores were inoculated. For each photomicrograph, we count the total number of spores and number of spores with rhizoid. The germination rate of each photomicrograph is calculated with the formula: number of spores with rhizoid/total number of spores x 100%.
For statistical analysis, we use Excel, Stata, and SPSS. To eliminate outliers, we perform a Grubbs test. To compare two data sets, we perform a Student’s t-test. To reduce false negative results, we perform power calculation using Stata. To compare three or more data sets, we perform a One-way ANOVA.
Recipes
- Liquid knop's medium (1 L)
Sterilize at 121 °C for 15 min using an autoclaveCa(NO3)2 0.5 g KNO3 0.125 g MgSO4 0.125 g KH2PO4 0.125 g FeCl3·6H2O 0.125 g Add 1 L H2O
Store liquid Knop’s medium at 4 °C - Solid knop’s medium (1 L)
Sterilize at 121 °C for 15 min using an autoclaveCa(NO3)2 0.5 g KNO3 0.125 g MgSO4 0.125 g KH2PO4 0.125 g FeCl3·6H2O 0.125 g Gellan Gum 3.0 g Add 1 L H2O
Pour 30 ml of medium into a dish to make solid Knop’s plate
Store solid Knop’s plate at 4 °C
Acknowledgments
This protocol was adapted from procedures published in Li et al. (2013) and Cao et al. (2010). We would like to thank Institute of Vegetables and Flowers, CAAS for providing greenhouse to grow A. capillus-veneris. The authors declare that there are no conflicts of interest or competing interests.
References
- Cao, J. G., Wang, Q. X., Dai, X. L. and Duckett, J. G. (2010). Ultrastructural observations of oogenesis in the fern Adiantum flabellulatum L. (Adiantaceae). Am Fern J 100: 93-102.
- Li, X., Fang, Y. H., Yang, J., Bai, S. N., and Rao, G. Y. (2013.) Overview of the morphology, anatomy, and ontogeny of Adinatum capillus-veneris: An experimental system to study the development of ferns. J Syst Evol 51(5): 499-510.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Yuan, Y., Yi, S., Jin, W. and Fang, Y. (2018). A Modified Approach for Axenic Cultivation of Spores of Fern Adiantum capillus-veneris L. with High Germination Rate. Bio-protocol 8(13): e2914. DOI: 10.21769/BioProtoc.2914.
Category
Plant Science > Plant developmental biology > Morphogenesis
Plant Science > Plant physiology > Axenic cultivation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










