- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Sociability and Social Novelty Preference Tests Using a U-shaped Two-choice Field
Published: Vol 8, Iss 10, May 20, 2018 DOI: 10.21769/BioProtoc.2853 Views: 11355
Reviewed by: Oneil G. BhalalaArnau Busquets-GarciaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1810 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1470 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1644 Views
Abstract
We developed sociability tests based on use of a U-shaped two-choice field to read out behavioral states of sociability in rodents. The U-shaped two-choice field is a modified open field that is partially partitioned with a wall projecting to the central point, resulting in two symmetrical rectangular fields, each containing closed and open square zones that together form a ‘U-shaped field’. The U-shaped two-choice field can be used to measure animal’s behavioral responses to two contrasting or similar options, such as (i) a social target versus an inanimate object, (ii) a new stranger versus an earlier stranger, and (iii) a novel animal (a non-mate) versus a cage-mate (a familiar animal). We describe detailed procedures for sociability tests for the above three behavioral test paradigms based on the U-shaped two-choice field.
Keywords: Social interactionBackground
Sociability is the behavioral disposition of being sociable with others. Sociability is defective in psychiatric disorders including depression, autism and schizophrenia. Defective sociability is regarded to be an important behavioral symptom representing a state of psychiatric illness (Hirschfeld et al., 2000; American Psychiatric Association., 2013; Green et al., 2015; Barak and Feng, 2016). Animal models have been used to investigate the neural mechanisms of sociability, as well as the neuropathological mechanisms of psychiatric illness. A variety of sociability tests have been developed to measure specific aspects of social behaviors of experimental animals, such as social interactions (Moy et al., 2004; Berton et al., 2006; McFarlane et al., 2008; Silverman et al., 2010), social communication using olfactory and visual cues or vocalization (Arakawa et al., 2008; Radyushkin et al., 2009; Scattoni et al., 2009; Yang and Crawley, 2009), social novelty preference and social memory (Moy et al., 2004; Kim et al., 2017a; Lee et al., 2017).
A sociability test using a U-shaped two-choice field was developed to read out behavioral states of sociability in mice (Seo et al., 2012). The U-shaped two-choice field is easily set up by partitioning an open field with a wall to the central point, so that the two symmetrical rectangular fields, each containing closed and open square zones, form a ‘U-shaped’ two-choice field (Park et al., 2014; Kim et al., 2015). Here, we describe how a U-shaped two-choice field can be used to measure animal’s behavioral response to two distinctive or contrasting options, namely (i) a social target versus an inanimate object or empty environment, (ii) an earlier stranger versus a new stranger, and (iii) a cage-mate (familiar one) versus a non-mate (unfamiliar one) (Seo et al., 2012; Park et al., 2014; Kim et al., 2015; 2016, 2017a, 2017b; Kim and Han, 2016a, 2016b, 2016c; Choi et al., 2015). Detailed procedures and the utility of the different sociability tests based on the U-shaped two-choice field are described below.
Materials and Reagents
- Latex examination gloves (Alliance: LGPF, Safeplus®)
- Paper towels
- 50-ml polypropylene conical tubes
- C57BL/6 mice
Note: Animals are described in detailed in Steps A1 and A2. - 70% ethanol
Note: The 70% ethanol and paper towels are used to remove mouse excrement from the floor and walls of the U-shaped field and grid cages before the start of each behaviour test sessions.
Equipment
- Two circular grid cages (Figure 1A)
Two circular grid cages (12 cm in diameter x 33 cm in height) made of tungsten wire.
Notes:- The grid cage needs to be high enough to prevent subject mice from climbing.
- It is helpful to cover the upper half of the grid cage with an overhead projector (OHP) film to prevent the subject mouse from climbing, and to add a white opaque partitioning in the middle of the grid cage to block the target mouse from climbing (Figure 1A).
- The grid cage needs to be high enough to prevent subject mice from climbing.
- The U-shaped two-choice field (Figures 1B and 1C)
The U-shaped two-choice field is a modified open field (45 cm in width x 45 cm in depth x 35 cm in height) that is partially partitioned with a wall (20 cm in width x 35 cm in height) to the central point, so that a ‘U-shaped’ field that contains two closed quadrants and two open quadrants of the same size is formed (Figures 1B and 1C).
Notes:- The U-shaped two-choice field is a modified open field made of cream colored FOAMEX panel (1 cm in thickness) (Expanded PVC; LG Ltd., Republic of Korea).
- The floor and walls of the U-shaped two-choice field are made of the same type of FOAMEX panel described above.
- Alternatives for the walls and floor might be workable, but reflective plastic materials or uncoated veneer plywood is not recommended. The floor should not be slippery.
- The U-field two-choice test is performed in an isolated room in the absence of potentially disrupting environmental factors. If an enclosed spacious room is not available, we recommend padding the outside walls of the U-shaped field with Styrofoam boards.
- The behavior test room is lit with 2 or 4 indirect lighting sources to achieve 20 lux on the floor of the U-shaped field.
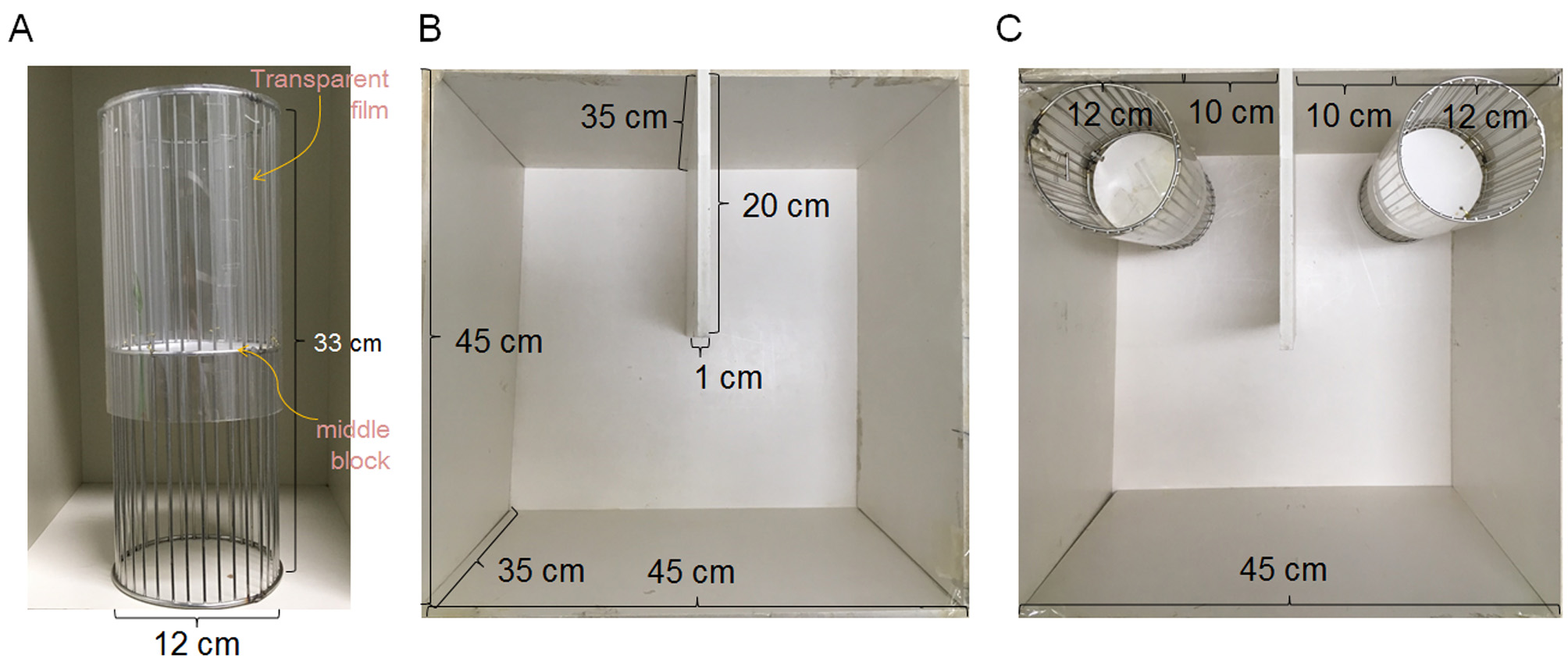
Figure 1. Photos showing the grid cages and U-shaped two-choice field. A. The circular grid cage. The upper half of the grid is covered with a transparent film. A white, opaque-circular partitioning is added in the middle of the grid cage. B. The open field is partially partitioned with a wall projecting to the center. C. A grid cage is placed in each outer corner of the closed quadrants making up the U-shaped two-choice field.
- The U-shaped two-choice field is a modified open field made of cream colored FOAMEX panel (1 cm in thickness) (Expanded PVC; LG Ltd., Republic of Korea).
- Brightness-adjustable indirect light sources
- Digital Lux meter (TES Electrical Electronic, catalog number: TES-1350A )
- White noise generator (HDT Korea, Genius mate)
- Sound level meter (TES Electrical Electronic, catalog number: TES-1330A )
- Stainless steel large forceps (20 cm in length) (Fine Science Tools, catalog number: 11000-20 ) with protective silicone tip guards (optional)
- Automatic recording systems
Software
- A computerized video tracking system (SMART, Panlab, Spain)
- GraphPad PRISM 6.0 software (GraphPad Software. Inc., San Diego, CA, USA) for data analysis
Procedure
Notes:
- All animals need to be handled following the local animal ethical protocols.
- All behavioral tests should be recorded using two independent recording systems: a computerized video tracking system (SMART, Panlab, Spain) and a Webcam camera (Logitech, Lausanne, Switzerland).
- Animals
- Subject mice
Seven-week-old male C57BL/6 mice were purchased from a local supplier (Daehan BioLink, Inc. Republic of Korea) one week before starting behavioral tests.
Notes: We recommend to include 10 mice (at least 8) in each experimental group to ensure high-fidelity behavioral measurements.- Following animals have also been tested and found to be workable.
- Seven-week-old male ICR mice purchased from a local vendor (OrientBio Inc. Republic of Korea).
- Seven-week-old male and female mice prepared from mating cages of C57BL/6 mice cultured in the institutional animal room facility.
- Seven-week-old male and female mice prepared from mating cages of transgenic or knockout mice carrying the C57BL/6 genetic background or the C57BL/6 x 129 hybrid background cultured in the institutional animal room facility.
- Seven-week-old male and female mice prepared from mating cages of ICR mice cultured in the institutional animal room facility.
- Seven-week-old male ICR mice purchased from a local vendor (OrientBio Inc. Republic of Korea).
- The source of experimental mice is potentially important and needs to be carefully considered.
- (Optional) Stress-induced depression models can be prepared to evalulate animals with deficits in social behaviors.
- Stress-induced depressive animals can be used as model animals that display sociability deficits.
- Mice treated with restraint for 2 h daily for 14 days exhibit depressive-like and anxiety-like behaviors that last for more than 3 months (Kim and Han, 2006; Seo et al., 2012; Park et al., 2014; Kim et al., 2015; 2016, 2017a, 2017b; Kim and Han, 2016a, 2016b, 2016c; Choi et al., 2015).
- Upon arrival, purchased mice are grouped according to experimental design, housed in pairs in each plastic cage, and allowed to acclimatize to the animal room facility (near the behavior test room if possible) without further disturbance for 5-7 days before starting behavioral tests. Mice prepared from one’s own animal room facility should be handled as above.
- Grouped mice should be housed ‘in pairs’ in a standard clear plastic cage with ad libitum access to food and water in the animal room at constant temperature (23 °C), humidity (50-60%), and a 12-h light/dark cycle (light on at 7 a.m.). Mice should not be housed singly in a cage unless this is an intended part of the experiments.
- Mice are individually restrained for 2 h daily in a ventilated 50-ml polypropylene conical tube and returned to their home cage. This restraint treatment should be repeated for 14 consecutive days, thus providing so called chronic restraint stress or the 2 h x 14 d RST (Kim and Han, 2006; Park et al., 2014). The 50-ml polypropylene conical tube should have 9-10 holes (2-3 mm in diameter) in the tube wall and lid for ventilation (Kim and Han, 2006).
- Stress-induced depressive animals can be used as model animals that display sociability deficits.
- Following animals have also been tested and found to be workable.
- Preparation of social targets: novel and familiar social targets
- Preparation of novel social targets
Normally, social targets should be of the same sex as the subject mice. Relative to subject mice, social targets may have the same genetic background (e.g., C57BL/6 social targets for C57BL/6 subject mice) at the same age.
Notes:- We recommend that social target mice not be smaller than subject mice.
- Social targets should be kept in pairs in a home cage.
- Social targets should never have been exposed to subject mice from the acclimation stage, except for cage mates.
- We recommend that social target mice not be smaller than subject mice.
- Preparation of familiar social targets (cage mates)
Cage mates should be prepared by housing two mice (or three if necessary) in a home cage for 2-3 days.
One of the cage mates should be used as social target, and the other as the subject mouse.
Notes:- Stranger (unfamiliar), cage mate (familiar), and subject mice should be of the same sex and may be of the same age.
- In studies with transgenic mice, familiar and unfamiliar mice should have the same genotype unless using different genotypes is a part of the experimental design.
- Subject mice should not be used as familiar social targets. Conversely, social targets should not be used as subject mice.
- If many social targets are needed, put three mice in a cage to make cage mates, and then use two of these mice as subject mice and the remaining one as the familiar social target.
- Stranger (unfamiliar), cage mate (familiar), and subject mice should be of the same sex and may be of the same age.
- Preparation of novel social targets
- Subject mice
- Sociability test procedures
- Preparation of sociability tests
- The behavior test room should be dimly lit with indirect lighting of 20 lux on the floor of the U-shaped field.
- Background noise should be masked by 65dB of white noise.
- Subject mice should be brought into the behavior testing room 20 min before starting the behavioral tests and placed in the same room during the test.
- The floor and walls of the U-shaped field and the grid cages should to be cleaned using 70% ethanol and paper towels before starting the next experiment.
- Behavioral tests should be conducted during the daylight cycle (9 a.m. to 3 p.m.).
- The behavior test room should be dimly lit with indirect lighting of 20 lux on the floor of the U-shaped field.
- Three types of sociability tests
- The U-shaped two-choice field may be used to test for (i) sociability and social interactions, (ii) social novelty recognition, and (iii) social novelty preferences.
- The sociability and social interaction test, and social novelty recognition test can be performed sequentially or separately, as described below.
2.1Habituation- The U-shaped two-choice field should be prepared with a grid cage at each outer corner of the closed quadrants (Figure 2A).
- The subject mouse should then be allowed to freely explore the U-shaped field equipped with empty grid cages on each side for 10 min (Figure 2B).
- The time spent and the trajectory taken in the zone and/or field are recorded.
- There should be no bias in exploration between two empty objects. If bias is present, this indicates that certain environmental cues are influencing mouse behavior; these should be eliminated.
- This habituation step is ‘a pre-target test’ that is required for the sociability test and social novelty preference test.
- The floor and walls of the U-shaped field and the grid cages should be cleaned using 70% ethanol and paper towels after each session of the behavior tests, but not between habituation and the sociability tests.
2.2Sociability and social interaction testIn this study, the sociability/social interaction test is defined as a test that measures the behavioral disposition of animals with regard to preferring a social target over an inanimate object (empty cage).- Immediately after habituation (2.1 section), the subject mouse is returned to its home cage for 2 min. While the subject mouse is in the home cage, the social target is loaded into a grid cage (Figure 2C).
- When the target mouse is stabilized in the grid cage for about 1 min, the subject mouse is placed in the middle open space of the U-shaped field (Figure 2D).
- The subject mouse is allowed to explore the U-shaped field containing a social target at one side and an inanimate grid cage at the other side for 10 min.
- The time spent and the trajectory taken in the zone and/or field, and the time spent exploring the social target are recorded. To assess a specific aspect of social interaction, sniffing time could be recorded. Sniffing is defined as when the subject mouse orients his head towards and within 2 cm of the grid cage.
- After each session, the served social target should be replaced by another mouse, and the served social target should be returned to his home cage for more than 10 min.
- Each social target can be reused as a social target, but less than three times in total.
- The floor and walls of the U-shaped field and the grid cages should be cleaned using 70% ethanol and paper towels after each session of the behavior test.
2.3Social novelty recognition testThe social novelty recognition test using the U-shaped field can be performed in the context of an earlier stranger (stranger 1) versus a new stranger (stranger 2).- In principle, this test requires an acute sensibility to distinguish between two similar strangers; a new stranger and a 10-min earlier stranger.
- The social novelty recognition test is useful to measure the behavioral disposition of animals in exploring social novelty, which requires short-term social memory.
- Immediately after the 10-min session of the sociability test (2.2 section, above), the subject mouse is returned to his home cage for 2 min.
- The social target in the sociability test (stranger 1; an earlier stranger) is placed in the grid cage on one side, and a new mouse (stranger 2; a new stranger) is placed in the grid cage on the opposite side, which was previously empty (Figure 2E).
- The subject mouse is placed to explore the U-shaped field for 10 min (Figure 2F).
- The time spent on each side, the trajectory taken, and the time spent exploring the two different social targets are recorded.
- Normally, C57BL/6 mice are able to distinguish an earlier vs. a new stranger and they spend more time exploring a new stranger.
- Stranger 1, stranger 2, and subject mice should all be of the same sex and should never have been in physical contact with each other.
- The floor and walls of the U-shaped field and the grid cages should be cleaned using 70% ethanol and paper towels after each session of the behavior tests.

Figure 2. Graphical presentation of the sociability and social interaction test, and social novelty recognition test. A-B. The habituation step. A photo showing the U-shaped two-choice field with an empty grid cage placed in each outer corner of the closed quadrants (A). For habituation, a subject mouse is allowed to freely explore the U-shaped two-choice field for 10 min (B). C-D. The sociability and social interaction test. A U-shaped field containing a social target on one side and an inanimate grid cage on the other side is prepared (C). The subject mouse is allowed to explore for 10 min (D). E-F. The social novelty recognition test. A U-shaped field containing a social stranger 1 (an earlier stranger, which was used as the social target in the sociability test) on one side and a social stranger 2 (a new stranger) on the other side is prepared (E). The subject mouse is allowed to explore for 10 min (F).2.4The social novelty preference testThe social novelty preference test in the context of a cage-mate (familiar) versus a non-mate (a stranger, unfamiliar) measures the behavioral disposition of the subject animal to seek a social novelty over a socially familiar target.- The U-shaped two-choice field is prepared with a grid cage in each outer corner of the closed quadrants (Figure 3A).
- The subject mouse is placed in the middle space of the U-shaped field and allowed to freely explore the U-shaped two-choice field for 10 min (Figure 3B). This procedure is identical to that described in the sociability test (Figures 2A and 2B), but this step is an independent one and required for the social novelty preference test.
- The time spent on each side, the trajectory taken, and the time spent exploring the social targets are recorded.
- Upon return of the subject mouse to the home cage for 2 min, a cage mate (a familiar mouse) is loaded into a grid cage at one side, while a novel (non-mate, unfamiliar) mouse is loaded into a grid cage at the other side (Figure 3C).
- The subject mouse is allowed to explore the U-shaped field containing a novel mouse (an unfamiliar mouse) and a cage mate (a familiar mouse) for 10 min.
- The time spent on each side, the trajectory taken, and the time spent exploring the social targets are recorded. To assess a specific aspect of social interaction, sniffing time could be recorded. Sniffing is regarded to occur when the subject mouse orients his head towards and within 2 cm of the grid cage.
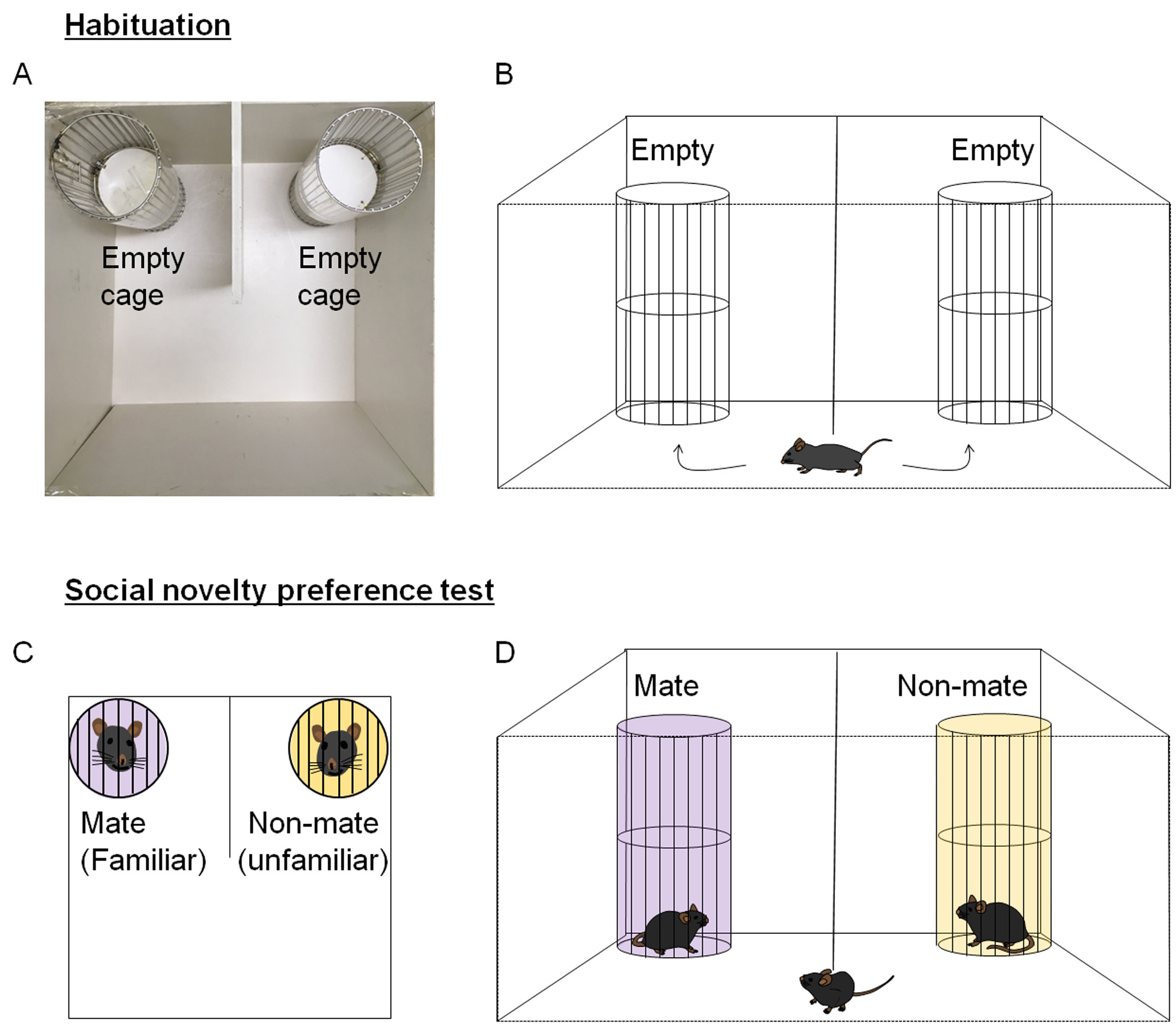
Figure 3. Graphical presentation of the novelty preference test. A-B. The habituation step. A photo showing the U-shaped two-choice field with an empty grid cage placed in each outer corner of the closed quadrants (A). The habituation step is accomplished by allowing a subject mouse to freely explore the U-shaped two-choice field for 10 min (B). C-D. The novelty preference test. A U-shaped field containing a cage mate (a familiar mouse) on one side and a non-mate (a novel unfamiliar mouse) on the other side is prepared (C). D. The subject mouse is allowed to explore the U-shaped field containing a cage mate (a familiar mouse) and a non-mate (a novel unfamiliar mouse) for 10 min.
Notes:
The social novelty preference test and the social novelty recognition test are similar, but measure different aspects of social novelty-seeking behaviors.- In the social novelty preference test, the subject mouse is presented with the choice of a fully habituated familiar animal and a social stranger, so this test is asking whether a subject mouse is more likely to explore a social stranger in the presence of familiar one or whether a subject mouse prefers to stay with the familiar aninimal in the presence of a social stranger.
- In the social novelty recognition test, the subject mouse is presented with two similar strangers, an earlier stranger and a new stranger, so this test is asking whether the subject mouse has the ability to distinguish between two similar targets and whether it prefers to explore the earlier stranger or new stranger. This task requires social recognition ability and short-term social recognition memory, although animal behavior in this test is also driven by social-novelty seeking.
- The U-shaped two-choice field may be used to test for (i) sociability and social interactions, (ii) social novelty recognition, and (iii) social novelty preferences.
- Preparation of sociability tests
Data analysis
- For the sociability test, the time spent exploring the target zone/field containing a social target divided by the time spent exploring the zone/field containing an inanimate object is regarded as the level of sociability (i.e., preference for a social target vs. inanimate object) (Figure 4C).
- Alternatively, sociability may be expressed as the relative proportion of the time spent exploring the target zone/field of mice relative to that of the habituation/pre-target test.
- Sociability may be also expressed as the sociability index (SI), which is defined as [the percent time spent in the target zone/field minus the percent time in the non-target zone/field] divided by [the percent time spent in the target zone/field plus the percent time in the non-target zone/ field] (Figure 4D).
- Social interaction level may be evaluated by assessing sniffing time. Sniffing is recorded when the subject mouse orients his head towards and within 2 cm of the grid cage.
- Alternatively, sociability may be expressed as the relative proportion of the time spent exploring the target zone/field of mice relative to that of the habituation/pre-target test.
- For the social novelty recognition test, the difference in time spent exploring a new stranger versus an earlier stranger can reflect short-term memory of an earlier stranger and the disposition for social exploration (Figures 4E and 4F).
- For the social novelty preference test, the time spent exploring an unfamiliar mouse versus that spent exploring a familiar mouse can be regarded as the level of the behavioural disposition for exploring and preferring social novelty (Figures 4G and 4H).
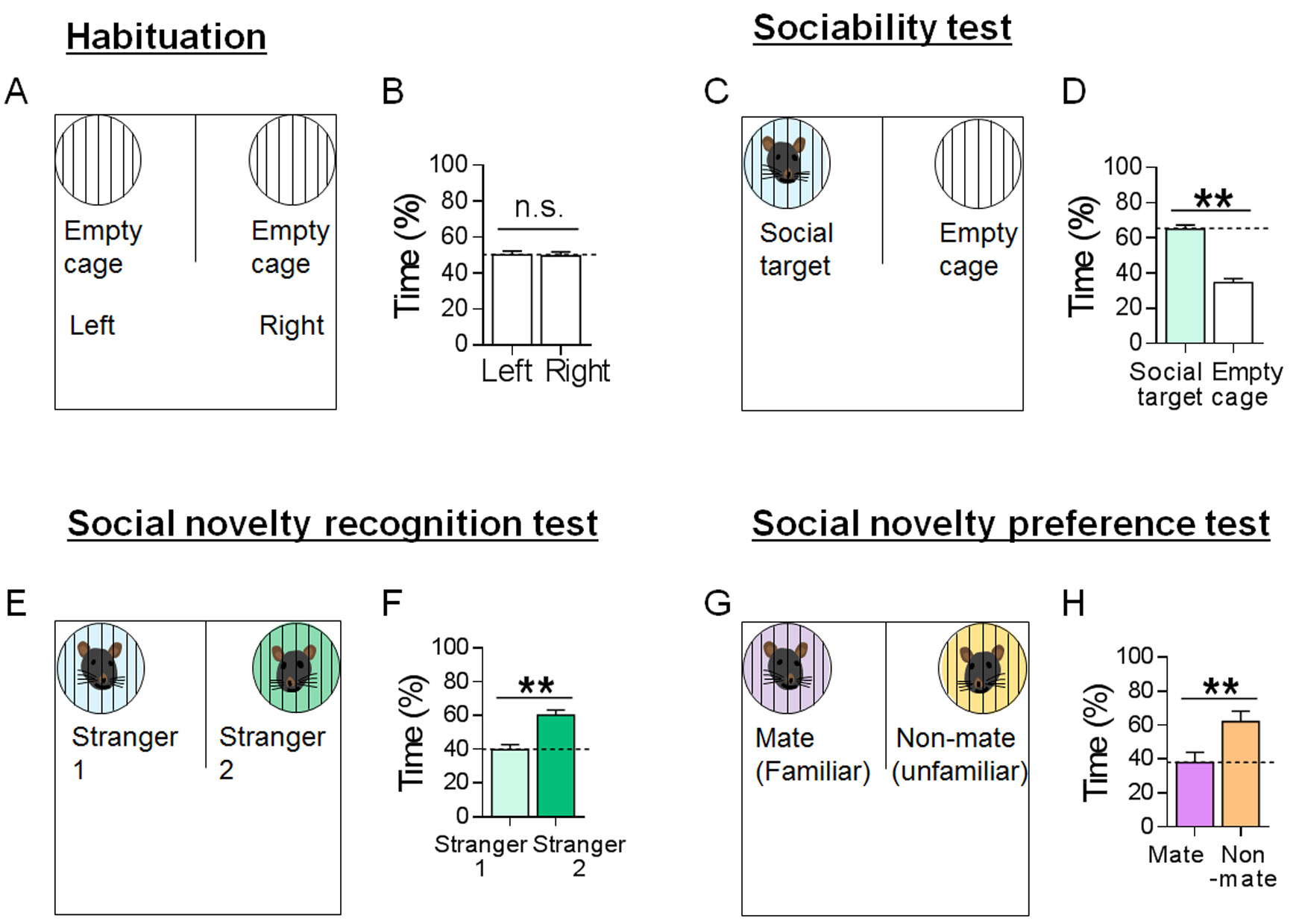
Figure 4. Data analyses of habituation, sociability test, social novelty recognition test and social novelty preference test results obtained using the U-shaped two-choice field. A-B. Habituation. Percent time spent by subject mice on each side of the U-shaped field for 10 min. Subject mice spent a similar amount of time (50.2% and 49.5%) on the left and right side of the U-shaped field above [left vs. right: t(52) = 0.2040, P = 0.8391]. C-D. The sociability test. Percent time spent by subject mice in the social target-containing field and empty cage-containing side field [t(16) = 8.440, P < 0.0001]. E-F. The social novelty recognition test. Percent time spent by subject mice in the stranger 1-containing field and stranger 2-containing field [t(32) = 0.8373, P = 0.0086]. G-H. The social novelty preference test. Percent time spent by subject mice in the mate-containing field and non-mate-containing field [t(14) = 2.806, P = 0.0140]. Data are presented as means ± SEM. * and ** denote differences between the two groups at P < 0.05 and P < 0.01, respectively. Student’s t-test. (Adapted from Park et al., 2014 and Lee et al., 2017)
Acknowledgments
This research was supported by a grant (2018R1A2B2001535) from the Ministry of Science, ICT and Future Planning, Republic of Korea. Sociability tests using a U-shaped two-choice field have been described in previous studies (Seo et al., 2012; Park et al., 2014; Kim et al., 2017a). The authors declare no competing financial interests.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th edition. American Psychiatric Publishing, Washington D.C.
- Arakawa, H., Blanchard, D. C., Arakawa, K., Dunlap, C. and Blanchard, R. J. (2008). Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev 32(7): 1236-1248.
- Barak, B. and Feng, G. (2016). Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat Neurosci 19(6): 647-655.
- Berton, O., McClung, C. A., Dileone, R. J., Krishnan, V., Renthal, W., Russo, S. J., Graham, D., Tsankova, N. M., Bolanos, C. A., Rios, M., Monteggia, L. M., Self, D. W. and Nestler, E. J. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311(5762): 864-868.
- Choi, J., Kim, J. E., Kim, T. K., Park, J. Y., Lee, J. E., Kim, H., Lee, E. H. and Han, P. L. (2015). TRH and TRH receptor system in the basolateral amygdala mediate stress-induced depression-like behaviors. Neuropharmacology 97: 346-356.
- Green, M. F., Horan, W. P. and Lee, J. (2015). Social cognition in schizophrenia. Nat Rev Neurosci 16(10): 620-631.
- Hirschfeld, R. M., Montgomery, S. A., Keller, M. B., Kasper, S., Schatzberg, A. F., Moller, H. J., Healy, D., Baldwin, D., Humble, M., Versiani, M., Montenegro, R. and Bourgeois, M. (2000). Social functioning in depression: a review. J Clin Psychiatry 61(4): 268-275.
- Kim, H., Lee, Y., Park, J. Y., Kim, J. E., Kim, T. K., Choi, J., Lee, J. E., Lee, E. H., Kim, D., Kim, K. S. and Han, P. L. (2017a). Loss of adenylyl cyclase Type-5 in the dorsal striatum produces autistic-like behaviors. Mol Neurobiol 54(10): 7994-8008.
- Kim, T. K., Kim, J. E., Park, J. Y., Lee, J. E., Choi, J., Kim, H., Lee, E. H., Kim, S. W., Lee, J. K., Kang, H. S. and Han, P. L. (2015). Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol Dis 79: 59-69.
- Kim, T. K., Lee, J. E., Kim, J. E., Park, J. Y., Choi, J., Kim, H., Lee, E. H. and Han, P. L. (2016). G9a-mediated regulation of OXT and AVP expression in the basolateral amygdala mediates stress-induced lasting behavioral depression and its reversal by exercise. Mol Neurobiol 53(5): 2843-2856.
- Kim, T. K. and Han, P. L. (2016a). Chronic stress and moderate physical exercise prompt widespread common activation and limited differential activation in specific brain regions. Neurochem Int 99: 252-261.
- Kim, T. K. and Han, P. L. (2016b). Physical exercise counteracts stress-induced upregulation of melanin-concentrating hormone in the brain and stress-induced persisting anxiety-like behaviors. Exp Neurobiol 25(4): 163-173.
- Kim, T. K. and Han, P. L. (2016c). Functional connectivity of basolateral amygdala neurons carrying orexin receptors and melanin-concentrating hormone receptors in regulating sociability and mood-related behaviors. Exp Neurobiol 25(6): 307-317.
- Kim, T. K., Kim, J. E., Choi, J., Park, J. Y., Lee, J. E., Lee, E. H., Lee, Y., Kim, B. Y., Oh, Y. J. and Han, P. L. (2017b). Local interleukin-18 system in the basolateral amygdala regulates susceptibility to chronic stress. Mol Neurobiol 54(7): 5347-5358.
- Kim, K. S. and Han, P. L. (2006). Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res 83(3): 497-507.
- Lee, Y., Kim, H., Kim, J. E., Park, J. Y., Choi, J., Lee, J. E., Lee, E. H. and Han, P. L. (2017). Excessive D1 dopamine receptor activation in the dorsal striatum promotes autistic-like behaviors. Mol Neurobiol.
- Moy, S. S., Nadler, J. J., Perez, A., Barbaro, R. P., Johns, J. M., Magnuson, T. R., Piven, J. and Crawley, J. N. (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav 3(5): 287-302.
- McFarlane, H. G., Kusek, G. K., Yang, M., Phoenix, J. L., Bolivar, V. J. and Crawley, J. N. (2008). Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav 7(2): 152-163.
- Park, J. Y., Kim, T. K., Choi, J., Lee, J. E., Kim, H., Lee, E. H. and Han, P. L. (2014). Implementation of a two-dimensional behavior matrix to distinguish individuals with differential depression states in a rodent model of depression. Exp Neurobiol 23(3): 215-223.
- Radyushkin, K., Hammerschmidt, K., Boretius, S., Varoqueaux, F., El-Kordi, A., Ronnenberg, A., Winter, D., Frahm, J., Fischer, J., Brose, N. and Ehrenreich, H. (2009). Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav 8(4): 416-425.
- Scattoni, M. L., Crawley, J. and Ricceri, L. (2009). Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev 33(4): 508-515.
- Seo, J. S., Park, J. Y., Choi, J., Kim, T. K., Shin, J. H., Lee, J. K. and Han, P. L. (2012). NADPH oxidase mediates depressive behavior induced by chronic stress in mice. J Neurosci 32(28): 9690-9699.
- Silverman, J. L., Yang, M., Lord, C. and Crawley, J. N. (2010). Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11(7): 490-502.
- Yang, M. and Crawley, J. N. (2009). Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci Chapter 8: Unit 8. 24.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lee, E., Park, J., Lee, Y. and Han, P. (2018). Sociability and Social Novelty Preference Tests Using a U-shaped Two-choice Field. Bio-protocol 8(10): e2853. DOI: 10.21769/BioProtoc.2853.
Category
Neuroscience > Behavioral neuroscience > Cognition
Neuroscience > Nervous system disorders > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link