- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Murine Hair Follicle Derived Stem Cell Transplantation onto the Cornea Using a Fibrin Carrier
Published: Vol 8, Iss 10, May 20, 2018 DOI: 10.21769/BioProtoc.2849 Views: 6623
Reviewed by: Vivien Jane Coulson-ThomasDongsheng JiangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Determining Bone-forming Ability and Frequency of Skeletal Stem Cells by Kidney Capsule Transplantation and Limiting Dilution Assay
Hitoshi Uchida [...] Wei Hsu
Mar 20, 2023 1701 Views

Preparation of Human Kidney Progenitor Cultures and Their Differentiation into Podocytes
Maria Elena Melica [...] Paola Romagnani
Aug 20, 2023 2274 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2411 Views
Abstract
The goal of this protocol is to establish a procedure for cultivating stem cells on a fibrin carrier to allow for eventual transplantation to the eye. The ability to transfer stem cells to a patient is critical for treatment for a variety of disorders and wound repair. We took hair follicle stem cells from the vibrissae of transgenic mice expressing a dual reporter gene under the control of the Tet-on system and the keratin 12 promoter (Meyer-Blazejewska et al., 2011). A clonal growth assay was performed to enrich for stem cells. Once holoclones formed they were transferred onto a fibrin carrier and cultivated to obtain a confluent epithelial cell layer. Limbal stem cell deficient (LSCD) mice were used as the transplant recipient in order to test for successful grafting and eventual differentiation into a corneal epithelial phenotype.
Keywords: HoloclonesBackground
Stem cells are widely used as a therapeutic tool, thus a means for delivery is essential. In fact, many researchers and companies are searching for the best way to deliver cells into the human body to optimize cell survival as well as integration into the host tissue. Injection methods have been widely used in animal models but often result in poor survival and integration. Techniques utilizing biomaterials and surgical devices are currently being employed. One technique that has been utilized to deliver stem cells is fibrin carriers. Fibrin gel is a degradable biopolymer that can adhere to native tissue allowing for cell attachment, migration and proliferation (Ehrbar et al., 2005). Fibrin gels have many advantages including biocompatibility, controlled degradation (Kjaergard et al., 1994; Sidelmann et al., 2000), uniform cell distribution and high cell seeding efficiency (Swartz et al., 2005). Fibrin gels have been utilized for treating skin burns (Pellegrini et al., 1999; Ronfard et al., 2000), junctional epidermolysis bullosa (Hirsch et al., 2017) and corneal damage (Pellegrini et al., 1997; Rama et al., 2010). The method described here uses a fibrin carrier to transplant hair follicle derived stem cells onto the ocular surface of a limbal stem cell-deficient mouse. Cell engraftment and differentiation was assessed for a 5-week period via fluorescent microscopy.
Materials and Reagents
- TISSEEL [Fibrin Sealant] (Baxter, catalog number: 1501261 )
- Insulin syringe (Fisher Scientific, catalog number: 14-829-1A)
Manufacturer: BD, catalog number: 329420 . - 6-well plates (Corning, Falcon®, catalog number: 353934 )
- Pipette tips (MidSci, Avant low binding tips)
- Transfer pipet
- HFSC discs
- Ethilon 10-0 nylon sutures (Ethicon, catalog number: 9006G )
- Microscope slides (Fisher Scientific, catalog number: 12-552-3 )
- Coverglass 22 x 50 (Fisher Scientific, catalog number: 12-548-5E )
- Thrombin
- C57BL/6 mice (THE JACKSON LABORATORY, catalog number: 000664 )
- K12rtTA/rtTA/tetO-cre/ROSAmTmG transgenic mice (see Notes)
- 0.9% saline (Fisher Scientific, catalog number: 23-535435 )
- BioGlo fluorescein sodium ophthalmic strips (Hub Pharmaceuticals, NDC 17238-900-11)
- Doxycycline chow (Custom Animal Diets, catalog number: AD3008 )
- Avastin (bevacizumab, Genentech, Inc.)
- Anti-inflammatory drops–Inflanefran forte (Allergan, NDC 11980-180)
- 16% paraformaldehyde (Electron Microscopy Sciences, catalog number: 15710 )
- Sodium borohydride (Sigma-Aldrich, catalog number: 71320 )
- DAPI (Thermo Fisher Scientific, InvitrogenTM, catalog number: D3571 )
- Sodium chloride (NaCl) (Fisher Scientific, catalog number: BP358-1 )
- Calcium chloride (CaCl2) (Acros Organics, catalog number: 349610025 )
- Dulbecco’s modified Eagle medium (DMEM) without calcium and magnesium (Thermo Fisher Scientific, GibcoTM, catalog number: 21068028 )
- Ham’s F12 Nutrient Mix (Thermo Fisher Scientific, GibcoTM, catalog number: 11765047 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10082147 )
- Human recombinant epidermal growth factor (Merck, catalog number: GF144 )
- L-Glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081 )
- Human corneal growth supplement (Thermo Fisher Scientific, GibcoTM, catalog number: S0095 )
- Penicillin-streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140148 )
- GibcoTM Amphotericin B (Thermo Fisher Scientific, catalog number: 15290026 )
- Sodium phosphate dibasic, anhydrous (Na2HPO4) (Sigma-Aldrich, catalog number: S7907 )
- Sodium phosphate monobasic, anhydrous (NaH2PO4) (Sigma-Aldrich, catalog number: S8282 )
- Analytical grade glycerol (Sigma-Aldrich, catalog number: G6279 )
- Mowiol 4-88 (Merck, catalog number: 475904 )
- Tris (Fisher Scientific, catalog number: BP152-1 )
- Ketamine/HCl 100 mg/ml (KetaJect; Henry Schein Animal Health, catalog number: 010177 )
- Xylazine AnaSed® 100 mg/ml (Santa Cruz Biotechnology, catalog number: sc-362949Rx )
- Fibrinogen solution (see Recipes)
- Thrombin solution (see Recipes)
- Stem cell media (see Recipes)
- 0.1 M Phosphate Buffer, pH 7.4 (see Recipes)
- Mowiol mounting medium (see Recipes)
- Ketamine/xylazine solution (see Recipes)
Equipment
- Algerbrush II Corneal rust ring remover (MicroSurgical Technology, catalog number: AM0100 )
- Inverted Fluorescence Microscope (Zeiss Observer Z1 with an apotome attachment) (ZEISS, model: AxioObserver Z1 )
- Suture Tying Forceps (Fine Science Tools, catalog number: 18025-10 )
- Microdissection scissors (Fine Science Tools, catalog number: 15000-00 )
- Epi-fluorescent stereomicroscope (ZEISS, model: Stemi SVII )
- BSL2 Laminar Flow Hood (Thermo Fisher Scientific, Thermo ScientificTM, model: 1300 Series A2 , catalog number: 1387)
- Hemocytometer (Hausser Scientific, catalog number: 3200 )
- CO2 Incubator (Thermo Fisher Scientific, Thermo ScienticTM, model: NAPCO Series 8000 WJ )
- Dissecting Scope (ZEISS, model: Stemi DV4 )
- Centrifuge (Hettich, model: Rotina 35 )
Software
- AxioVison 4.7
Procedure
- Preparation of fibrin carrier
- Thaw the TISSEEL Fibrin Sealant (2-ml pre-filled syringe) at 37 °C.
- Prepare diluted fibrin by adding 1 ml of Sealer Protein (fibrinogen) entire contents of the syringe to 1.16 ml of fibrinogen solution (Recipe 1).
- Prepare thrombin mix by adding 1 ml of thrombin (entire contents of the syringe) to 9 ml of thrombin solution (Recipe 2).
- Prepare diluted thrombin by adding 1.5 ml of thrombin mix (from Step A3) to 25 ml thrombin solution.
- To one well of a 6-well plate add 0.75 ml of diluted thrombin (from Step A3). Ensure that the diluted thrombin solution covers the entire well evenly.
- To the same well add 0.75 ml diluted fibrin. Add dropwise to the well quickly mixing with a pipette tip.
- Allow gel to set at room temperature. The final concentration of fibrinogen and thrombin is ~10 mg/ml and 3 IU/ml, respectively. The gel will set in approximately 5 min. Once the gel has set proceed to the cultivation of the cells.
- Thaw the TISSEEL Fibrin Sealant (2-ml pre-filled syringe) at 37 °C.
- Cultivation on a fibrin carrier
- Seed hair follicle stem cells derived from a dual reporter mouse at 1 x 105 cells/ml onto a fibrin carrier and cultivate for 3 days in stem cell media (Recipe 3) at 37 °C, 5% CO2. A total volume of 2 ml is used for each well. Cells can be visualized using phase contrast microscopy (Figure 1)
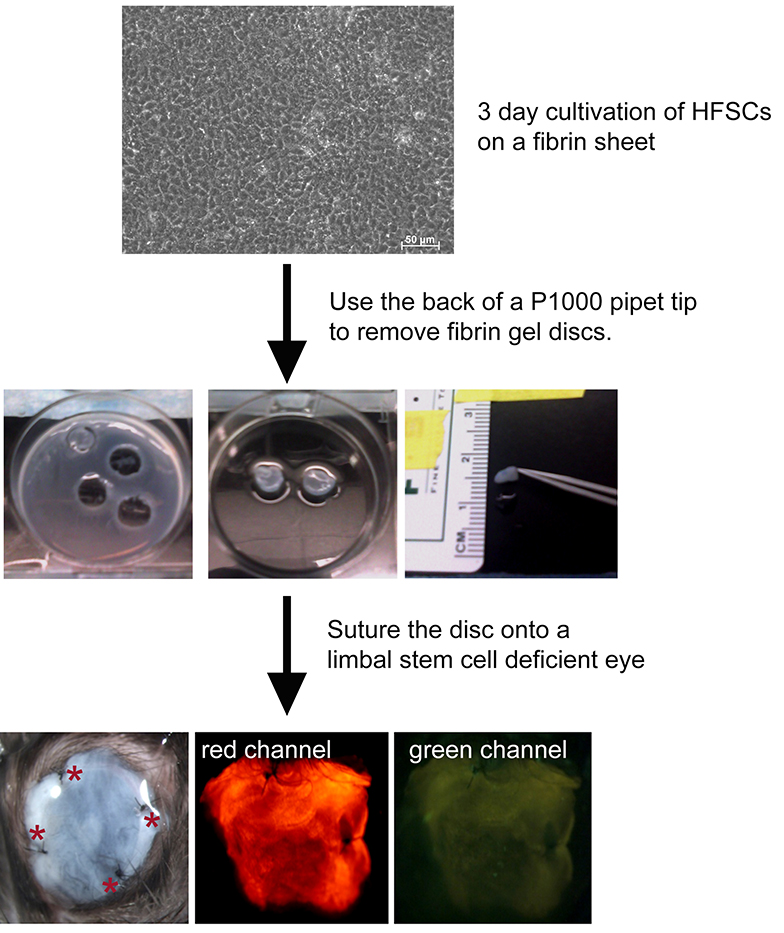
Figure 1. Scheme showing transplant of a fibrin carrier containing bulge derived hair follicle stem cells. Discs are prepared from hair follicle stem cells (HFSCs) from a dual reporter mouse cultivated for 3 days on a fibrin sheet by using the back of a pipet tip as a “cookie cutter”. Discs are then sutured to the eye of a limbal stem cell deficient mouse. Four sutures (red asterisks) are placed. Cells fluoresce red but not green as they do not yet express the corneal epithelial differentiation marker, Krt12.
- Seed hair follicle stem cells derived from a dual reporter mouse at 1 x 105 cells/ml onto a fibrin carrier and cultivate for 3 days in stem cell media (Recipe 3) at 37 °C, 5% CO2. A total volume of 2 ml is used for each well. Cells can be visualized using phase contrast microscopy (Figure 1)
- Total limbal stem cell deficiency (Figure 2)
- Anesthetize 6- to 8-week old C57BL/6 mice with ketamine (90 mg/kg) and xylazine (13 mg/kg) (Recipe 6) via intraperitoneal injection using a 28-G insulin syringe.
- Generate total limbal stem cell deficiency by removing the entire corneal and limbal epithelium using an Algerbrush II corneal rust ring remover with a 0.5 mm burr (Note 6). Hair follicle stem cell transplantation (see below) should immediately follow the removal of epithelial cells.
- Verify complete removal of corneal and limbal epithelium using fluorescein stain.
- Dip the tip of a fluorescein sodium ophthalmic strip in 0.9% saline for 20 times.
- Using a transfer pipet, add a drop of the fluorescein stain onto the eye. Let sit 30 sec.
- Extensively wash in 0.9% saline.
- Image using a fluorescent stereomicroscope.
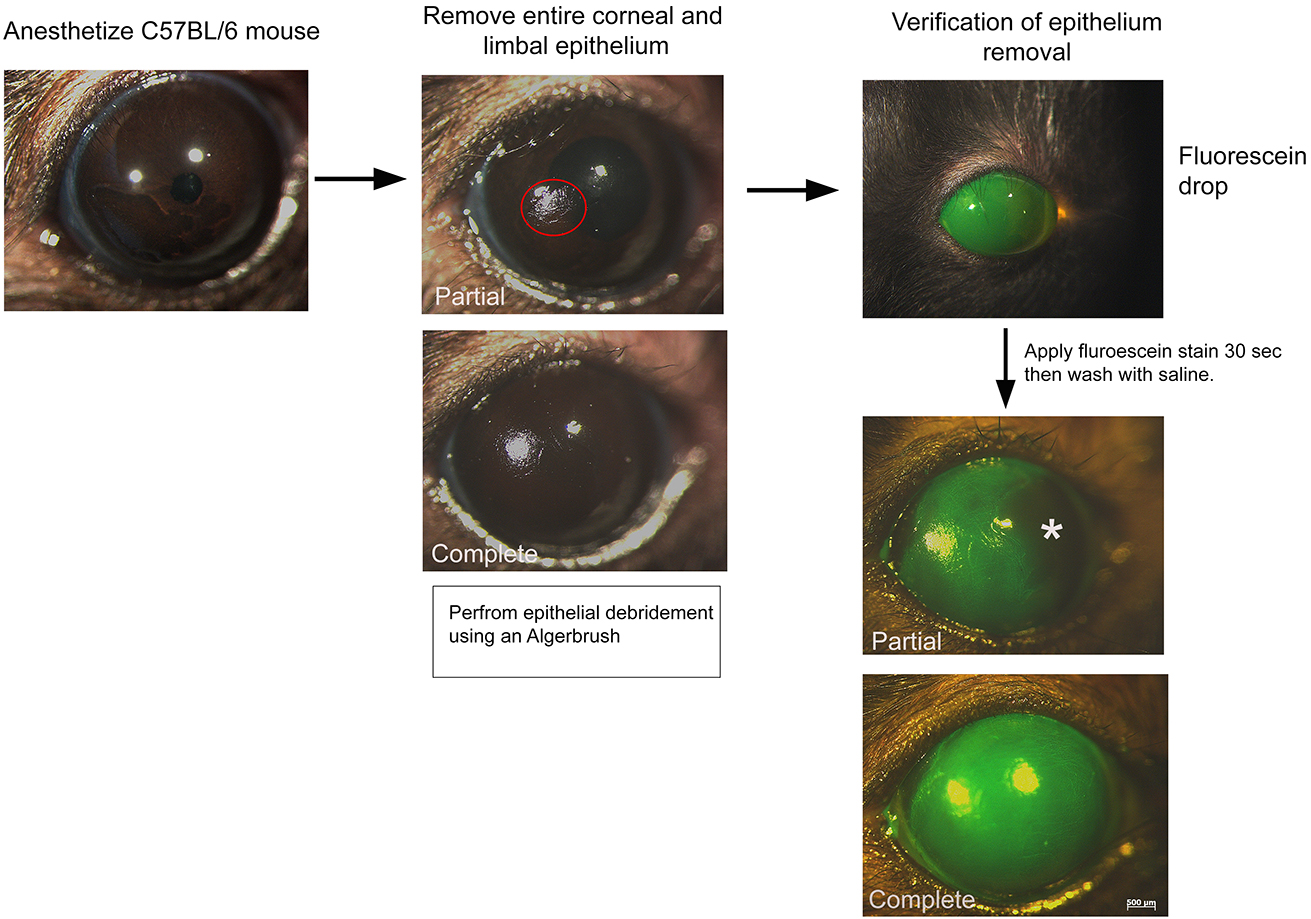
Figure 2. Scheme of limbal stem cell deficiency. An algerbrush is used to remove the entire corneal and limbal epithelium. The red circle depicts partial debridement. Fluorescein stain can be used to verify the complete removal of the epithelium. Note the absence of fluorescein stain in the partial removal (asterisk). - Dip the tip of a fluorescein sodium ophthalmic strip in 0.9% saline for 20 times.
- Anesthetize 6- to 8-week old C57BL/6 mice with ketamine (90 mg/kg) and xylazine (13 mg/kg) (Recipe 6) via intraperitoneal injection using a 28-G insulin syringe.
- Hair follicle stem cell transplantation
- Prepare ~7 mm fibrin gel containing HFSC discs by using the back of a P1000 pipet tip (see Figure 1). Place the discs into PBS (Note 5) using forceps (Figure 1).
- Suture an approximately 7 mm fibrin carrier containing HFSC onto the surface of a limbal stem cell-deficient mouse eye with the stem cells facing the recipient’s basement membrane. A suture was placed superiorly, inferiorly, nasally and temporally (Figure 1).
- Following surgical recovery, administer anti-inflammatory eye drops for the first five days followed by Avastin eye drops for the next 7 days. Eye drops should be administered once a day.
- Prepare ~7 mm fibrin gel containing HFSC discs by using the back of a P1000 pipet tip (see Figure 1). Place the discs into PBS (Note 5) using forceps (Figure 1).
- Conversion to corneal epithelial cells
- Mice are fed doxycycline chow (1 g/kg) ad libitum until sample collection at 3 days, 1 week, 2 weeks, 3 weeks and 5 weeks.
- Euthanize mice and enucleate the eyes at the sample collection time points and fix in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 (Recipe 4) at 4 °C overnight followed by PBS washes.
- Dissect the cornea using microdissection scissors.
- Treat the corneas with 0.2% sodium borohydride (Note 7) for 30 min at room temperature followed by washing in 0.1 M phosphate buffer, pH 7.4.
- Stain corneas with 4’6-diamidino-2-phenylindole (DAPI, 1 μg/ml), mount onto a microscope slide using cover glass and Mowiol medium (Recipe 5) and visualize using a Zeiss Observer Z1 microscope with an apotome attachment.
- Mice are fed doxycycline chow (1 g/kg) ad libitum until sample collection at 3 days, 1 week, 2 weeks, 3 weeks and 5 weeks.
Data analysis
AxioVison 4.7. Z-stack images in the red (membrane tomato red), green (membrane EGFP) and blue (DAPI) channels were taken for each collection time point. Conversion of red to green was noted at each of the time points (Figure 3).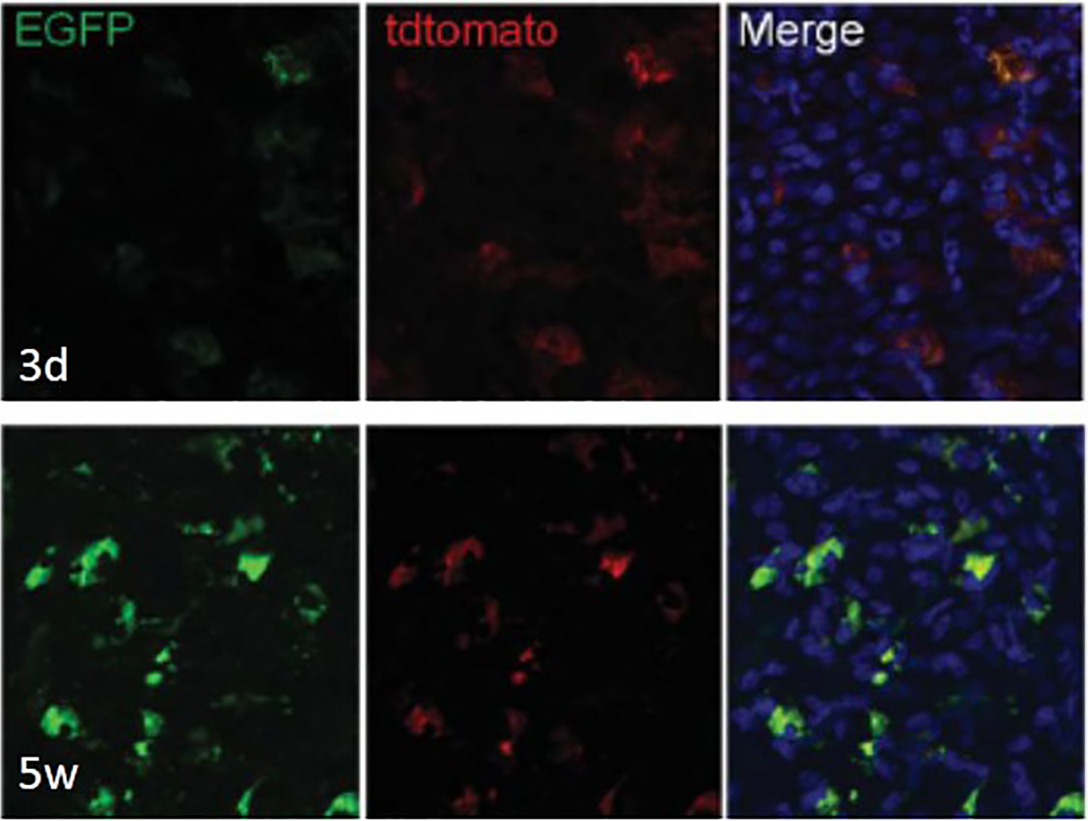
Figure 3. Engraftment and differentiation of the hair follicle bulge-derived stem cells into corneal epithelial cells. Single color images taken from a Z-stack depicting the cellular localization of membrane tomato red and membrane EGFP at 3 days and 5 weeks post-transplantation. Magnification x200. Reprinted with permission from Meyer-Blazejewska et al., 2011.
Notes
- Preparation of the fibrin gel and cultivation of the HFSC should be performed in a class II biological safety cabinet.
- Various dimensions of the fibrin carrier can be made as long as the ratio of diluted thrombin and diluted fibrin remain 1:1.
- All cell counts were performed using a hemocytometer.
- All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. Genetically modified mouse lines Krt12rtTA (Chikama et al., 2005), TetO-cre (Perl et al., 2002) and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J(ROSAmTmG) (Muzumdar et al., 2007) have been previously described. Compound transgenic mice were generated by breeding individual mouse lines to create K12rtTA/rtTA/tetO-cre/ROSAmTmG. This dual reporter mouse model uses the keratin 12 promoter (corneal epithelium-specific) to drive the expression of the Tet-on system. In conjunction with doxycycline and cre, the membrane tomato red hair follicle stem cells will turn green if they have differentiated into corneal epithelial cells.
- When creating and transferring the fibrin gel discs be sure to handle them with care as to avoid damaging the cells. Additionally, be sure the discs are placed in PBS with the HFSC facing upwards.
- When removing the epithelium with the Algerbrush care should be taken to avoid damaging the basement membrane. This can be done by holding the Algerbrush at a 45° angle and moving it in a circular, sweeping motion. Use only gentle pressure as the epithelium is relatively easy to remove.
- Care should be taken when handling sodium borohydride as it poses physical and health hazards.
Recipes
- Fibrinogen solution
10% NaCl
100 mM CaCl2
0.9% NaCl
ddH2O - Thrombin solution
10% NaCl
100 mM CaCl2
ddH2O - Stem cell media
3 parts DMEM/High glucose without Ca2+ or Mg2+
1 part Ham’s F12
10% FBS
10 ng/ml EGF
500 mg/L L-glutamine
0.4 mM calcium chloride
1x human corneal growth supplement
10,000 U/ml penicillin
10,000 μg/ml streptomycin
25 μg/ml amphotericin B - 0.1 M Phosphate Buffer, pH 7.4
76 mM Na2HPO4 (anhydrous)
27 mM NaH2PO4 (anhydrous) - Mowiol mounting medium
24 g analytical grade glycerol
9.6 g Mowiol 4-88
24 ml ddH2O
48 ml 0.2 M Tris-HCl buffer, pH 8.5 - Ketamine/xylazine solution
2 ml of ketamine (100 mg/ml)
0.25 ml xylazine (100 mg/ml)
7.75 ml PBS
Acknowledgments
This study was supported in part by grants from the NIH/NEI EY011845, Ohio Lions Eye Research Foundation to W.W.K. Authors do not have any conflicts of interest.
References
- Chikama, T., Hayashi, Y., Liu, C. Y., Terai, N., Terai, K., Kao, C. W., Wang, L., Hayashi, M., Nishida, T., Sanford, P., Doestchman, T. and Kao, W. W. (2005). Characterization of tetracycline-inducible bitransgenic Krt12rtTA/+/tet-O-LacZ mice. Invest Ophthalmol Vis Sci 46(6): 1966-1972.
- Ehrbar, M., Metters, A., Zammaretti, P., Hubbell, J. A. and Zisch, A. H. (2005). Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J Control Release 101(1-3): 93-109.
- Hirsch, T., Rothoeft, T., Teig, N., Bauer, J. W., Pellegrini, G., De Rosa, L., Scaglione, D., Reichelt, J., Klausegger, A., Kneisz, D., Romano, O., Secone Seconetti, A., Contin, R., Enzo, E., Jurman, I., Carulli, S., Jacobsen, F., Luecke, T., Lehnhardt, M., Fischer, M., Kueckelhaus, M., Quaglino, D., Morgante, M., Bicciato, S., Bondanza, S. and De Luca, M. (2017). Regeneration of the entire human epidermis using transgenic stem cells. Nature 551(7680): 327-332.
- Kjaergard, H. K. and Weis-Fogh, U. S. (1994). Important factors influencing the strength of autologous fibrin glue; the fibrin concentration and reaction time--comparison of strength with commercial fibrin glue. Eur Surg Res 26(5): 273-276.
- Meyer-Blazejewska, E. A., Call, M. K., Yamanaka, O., Liu, H., Schlotzer-Schrehardt, U., Kruse, F. E. and Kao, W. W. (2011). From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 29(1): 57-66.
- Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. and Luo, L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45(9): 593-605.
- Pellegrini, G., Ranno, R., Stracuzzi, G., Bondanza, S., Guerra, L., Zambruno, G., Micali, G. and De Luca, M. (1999). The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation 68(6): 868-879.
- Pellegrini, G., Traverso, C. E., Franzi, A. T., Zingirian, M., Cancedda, R. and De Luca, M. (1997). Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349(9057): 990-993.
- Perl, A. K., Wert, S. E., Nagy, A., Lobe, C. G. and Whitsett, J. A. (2002). Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A 99(16): 10482-10487.
- Rama, P., Matuska, S., Paganoni, G., Spinelli, A., De Luca, M. and Pellegrini, G. (2010). Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 363(2): 147-155.
- Ronfard, V., Rives, J. M., Neveux, Y., Carsin, H. and Barrandon, Y. (2000). Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix. Transplantation 70(11): 1588-1598.
- Sidelmann, J. J., Gram, J., Jespersen, J. and Kluft, C. (2000). Fibrin clot formation and lysis: basic mechanisms. Semin Thromb Hemost 26(6): 605-618.
- Swartz, D. D., Russell, J. A. and Andreadis, S. T. (2005). Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol 288(3): H1451-1460.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Call, M., Meyer, E. A., Kao, W. W., Kruse, F. E. and Schloetzer-Schredhardt, U. (2018). Murine Hair Follicle Derived Stem Cell Transplantation onto the Cornea Using a Fibrin Carrier. Bio-protocol 8(10): e2849. DOI: 10.21769/BioProtoc.2849.
Category
Stem Cell > Adult stem cell > Epithelial stem cell
Stem Cell > Adult stem cell > Cell transplantation
Cell Biology > Cell Transplantation > Allogenic Transplantation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








