- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Analysis of Ubiquitin-like Protein Modification in Archaea
Published: Vol 8, Iss 10, May 20, 2018 DOI: 10.21769/BioProtoc.2845 Views: 7007
Reviewed by: Elizabeth LibbySrujana Samhita YadavalliAgnès Groisillier

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

H2 Production from Methyl Viologen–Dependent Hydrogenase Activity Monitored by Gas Chromatography
Nuttavut Kosem
Dec 5, 2023 1758 Views

Monitoring Protein Stability In Vivo Using an Intein-Based Biosensor
John S. Smetana [...] Christopher W. Lennon
Apr 20, 2025 1575 Views

Endo-1,4-β-D-xylanase Assay Using Azo-Xylan and Variants Thereof
Luca Bombardi [...] Salvatore Fusco
Apr 20, 2025 1916 Views
Abstract
The ubiquitin-like (Ubl) protein is widely distributed in Archaea and involved in many cellular pathways. A well-established method to reconstitute archaeal Ubl protein conjugation in vitro is important to better understand the process of archaeal Ubl protein modification. This protocol describes the in vitro reconstitution of Ubl protein modification and following analysis of this modification in Haloferax volcanii, a halophilic archaeon serving as the model organism.
Keywords: ArchaeaBackground
The process by which ubiquitin (Ub) is covalently attached to target proteins is termed ubiquitination, which controls an enormous range of cellular process in eukaryotic cells (Glickman and Ciechanover, 2002; Komander and Rape, 2012). Ubiquitination is catalyzed by a cascade of enzymes including an Ub-activating enzyme (E1), Ub-conjugating enzymes (E2s), and Ub ligases (E3s). In vitro reconstitution of ubiquitination is a useful assay to determine the specificity between enzymes or between E3s and protein substrates (Zhao et al., 2012). In Archaea, the Ubl protein SAMP adopts a Ub-fold and is isopeptide-linked to protein targets catalyzed by an E1-like enzyme UbaA [reviewed in Maupin-Furlow, (2014)]. While E1 homologs are widespread in Archaea, canonical E2 or E3 enzymes are not predicted in most Archaea based on primary sequence comparisons. Our recent study of Haloferax volcanii, shows methionine sulfoxide reductase A (MsrA) is needed for Ubl protein modification (sampylation) together with UbaA under a mild oxidative condition in vivo and in vitro (Fu et al., 2017). Here, we describe a detailed in vitro protocol to reconstitute and analyze MsrA-dependent sampylation.
Materials and Reagents
- Gloves (Fisherbrand, Fisher Scientific, catalog number: 19-130-1597C )
- Sterile toothpicks (Royal Paper Products, Inc., item number: R820)
- Ampac 500 series SealPAK heavy duty pouches (10.2 x 15.2 cm) (Fisher Scientific, catalog number: 01-812-25D) (seal Western blot membrane with CDP-Star or ECL prime reagents in pouches using colored labeling tape)
Manufacturer: Ampac, catalog number: 50024 . - Sterile loop (Fisherbrand, Fisher Scientific, catalog number: 22-363-599 )
- Fernbach flasks (2.8 L, wide mouth) (Corning, PYREX®, catalog number: 4420-2XL )
- Baffled culture flasks (250 ml) (DWK Life Sciences, Kimble, catalog number: 25630-250 )
- Aluminum foil (Fisherbrand, Fisher Scientific, catalog number: 01-213-105 ) (used to wrap items for autoclaving)
- Sterile polystyrene disposable serological 10 ml pipets with magnifier stripe (Fisherbrand, Fisher Scientific, catalog number: 13-678-11E )
- Polypropylene round-bottom centrifuge tubes, 50 ml (Nalgene, Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 3119-0050 , item number: UX-06327-47)
- Nalgene rapid-flow sterile disposable bottle top 0.2 µm filters with surfactant-free cellulose acetate (SFCA) membrane (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 290-3320 )
- HisTrap HP column (5 ml) (GE Healthcare, catalog number: 17524802 )
- StrepTrap HP column (1 ml) (GE Healthcare, catalog number: 29048653 )
- 13 x 100 mm2 culture tubes (Fisher Scientific, catalog number: 14-961-27 )
- Borosilicate glass tubes with plain end (13 x 100 mm) (Fisherbrand, Fisher Scientific, catalog number: 14-961-27 ) (used for cell culture)
- Amicon Ultra-4 centrifugal filter unit with Ultracel-3 membrane (NMWL 3 kDa) (Merck, catalog number: UFC800308 )
- Superdex 75 10/300 GL column (GE Healthcare, catalog number: 17517401 )
- Surfactant-free cellulose acetate (SFCA) membrane syringe filters (28-mm membrane with 0.2, 0.45 and 0.8 µm pore sizes in acrylic housing) (Corning, catalog numbers: 431219 , 431220 , and 431221 )
- SnakeSkin dialysis tubing (3.5K MWCO, 22 mm I.D.) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 68035 )
- 1.5 ml microcentrifuge tubes (Fisherbrand, Fisher Scientific, catalog number: 02-681-320 )
- 2.0 ml microcentrifuge tubes (Fisherbrand, Fisher Scientific, catalog number: 02-681-321 )
- Zeba Spin Desalting Columns (7K MWCO) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 89882 )
- Amersham Hybond P 0.45 polyvinylidene difluoride (PVDF) membrane (GE Healthcare, catalog number: 10600023 )
- Disposable plastic cuvettes (Fisherbrand, Fisher Scientific, catalog number: 149-551-27 )
- X-ray film (RPI, catalog number: 248300 )
- Rack LTS tips (‘P20’ 2-20 µl, ‘P200’ 20-200 µl, ‘P1000’ 100-1,000 µl) (Mettler-Toledo, Rainin, catalog numbers: 17001865 , 17001863 and 17001864 )
- 10 ml Luer-Lok syringe (BD, catalog number: 309695 )
- Kimwipes Delicate Task Wipers, 1-Ply (KWCC, Kimberly-Clark, catalog number: 34155 )
- Colored labeling tape, rainbow pack (Fisherbrand, Fisher Scientific, catalog number: 15-901-10R )
- Four square cassettes (8 x 10") (Fisher Scientific, catalog number: FBXC-810 ) (for exposure of Western blot membrane to X-ray film)
- Glass storage/media bottles (DWK Life Sciences, Kimble, catalog numbers: 14395-500 for 500 ml and 14395-1000 for 1,000 ml)
- Polypropylene griffin low-form plastic beakers (Thermo Fisher Scientific, Thermo ScientificTM, catalog numbers: 1201-4000 for 2,000 ml and 1201-1000 for 1,000 ml)
- Polyvinyl wrapping film (Fisherbrand, Fisher Scientific, catalog number: 15-610 ) (can store SDS-PAGE gels wrapped in Kimwipes that are moistened with deionized water and further wrapped with polyvinyl film for up to 2 weeks at 4 °C prior to electrophoresis)
- Polystyrene cuvettes (1.5 ml capacity) (Fisherbrand, Fisher Scientific, catalog number: 14-955-127 ) (used for assays in visible spectral range, 340 to 750 nm)
- Polypropylene plastic graduated cylinders (Nalgene, Thermo Fisher Scientific, Thermo ScientificTM, catalog numbers: 3664-0050 , 3664-0250 , and 3664-1000 )
- Polypropylene closures (DWK Life Sciences, Kimble, catalog number: 73660-13 ) (used with 13 mm O.D. plain-end culture tubes)
- Petri dishes with clear lid (Fisherbrand, Fisher Scientific, catalog number: FB0875712 )
- Cells
- Haloferax volcanii LR03 (Δsamp1 Δsamp2 Δsamp3 ΔmsrA ΔubaA) carrying plasmid pJAM3010 (a plasmid encoding msrA-strepII under control of the P2rrnA constitutive promoter) (Fu et al., 2017) or pJAM1209 (a plasmid encoding his6-ubaA under control of the P2rrnA constitutive promoter) (Hepowit et al., 2016) or without carrying any plasmid
Note: H. volcanii strains and plasmids are available upon request. - Escherichia coli Rosetta (DE3) (Novagen, Merck, catalog number: 70954-3 ) carrying plasmid pJAM3200 (a plasmid encoding msrA-strepII under control of T7 promoter) from the Maupin-Furlow lab (Fu et al., 2017) or plasmid pJAM1132 (a plasmid encoding flag-his6-samp2 under control of T7 promoter) from the Maupin-Furlow lab (Hepowit et al., 2016)
Note: Plasmids are available from the Maupin-Furlow lab upon request.
- Haloferax volcanii LR03 (Δsamp1 Δsamp2 Δsamp3 ΔmsrA ΔubaA) carrying plasmid pJAM3010 (a plasmid encoding msrA-strepII under control of the P2rrnA constitutive promoter) (Fu et al., 2017) or pJAM1209 (a plasmid encoding his6-ubaA under control of the P2rrnA constitutive promoter) (Hepowit et al., 2016) or without carrying any plasmid
- MsrA-StrepII (purified as per procedure outlined below)
- His6-UbaA (purified as per procedure outlined below)
- Deionized H2O (for details see Equipment item number 27)
- Glycerol (Sigma-Aldrich, catalog number: G5516 )
- Novobiocin (Sigma-Aldrich, catalog number: N1628 )
- Kanamycin sulfate (Fisher BioReagents, Fisher Scientific, catalog number: BP906-5 )
- Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Fisher BioReagents, Fisher Scientific, catalog number: BP1620-1 )
- d-Desthiobiotin (Sigma-Aldrich, catalog number: D1411-500MG )
- Imidazole, ACS Reagent, ≥ 99% titration (Sigma-Aldrich, catalog number: I2399 )
- Coomassie Brilliant Blue R-250 (Bio-Rad Laboratories, catalog number: 1610436 )
- Pierce Bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 23225 )
- Albumin, bovine (BSA) (lyophilized powder, ≥ 96% purity by agarose gel electrophoresis) (Sigma-Aldrich, catalog number: A2153 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Fisher Chemical, Fisher Scientific, catalog number: M35-12 )
- Bortezomib proteasome inhibitor (free base, > 99% purity) (LC Laboratories, catalog number: B-1408 )
- DNase I from bovine pancreas (Sigma-Aldrich, catalog number: D4263-1VL ), a standardized vial containing 2,000 Kunitz units of DNase I (Sigma-Aldrich, catalog number: D4527 ) at ≥ 0.25 mg total protein
- Dimethylformamide (DMF) (Fisher BioReagents, Fisher Scientific, catalog number: BP1160-500 )
- Sodium chloride (NaCl) (Fisher Chemical, Fisher Scientific, catalog number: S642-12 )
- Adenosine 5’-triphosphate (ATP), disodium salt hydrate, 98% (ACROS Organics, catalog number: 102800100 )
- Dimethyl sulfoxide (DMSO), molecular biology grade (Sigma-Aldrich, catalog number: D8418-50ML ) (for in vitro assays)
- DMSO, Certified ACS (Fisher Chemical, Fisher Scientific, catalog number: D128-1 ) (for culturing cells)
- Dithiothreitol (DTT) (Fisher BioReagents, Fisher Scientific, catalog number: BP172-5 )
- Antibodies
- Strep-tag II monoclonal antibody (in mouse) (QIAGEN, catalog number: 34850 )
- Anti-His IgG2 monoclonal antibody (in mouse) (GE Healthcare, catalog number: 27471001 )
- HRP-conjugated His6 monoclonal antibody (Proteintech, catalog number: HRP-66005 ) (anti-His6 antibody replaced the discontinued GE Healthcare product listed above)
- Goat anti-mouse IgG (whole molecule)-alkaline phosphatase-linked antibody (Sigma-Aldrich, catalog number: A5153-1ML )
- Alkaline phosphatase-linked anti-Flag M2 monoclonal antibody (Sigma-Aldrich, catalog number: A9469-2MG )
- Strep-tag II monoclonal antibody (in mouse) (QIAGEN, catalog number: 34850 )
- Nonfat dry milk (instant, powdered) (Publix, Lakeland, FL item)
- Tropix CDP-Star chemiluminescent substrate (12.5 mM concentrate) (Applied BioSystems, Thermo Fisher Scientific, InvitrogenTM, catalog number: T2304 )
- cOmplete His-tag purification resin (Sigma-Aldrich, Roche Diagnostics, catalog number: 5893682001 )
- Sodium phosphate monobasic monohydrate (H2NaO4P·H2O) (Fisher Chemical, Fisher Scientific, catalog number: S369-1 )
- Potassium phosphate monobasic (KH2PO4) (Fisher Chemical, Fisher Scientific, catalog number: P285-500 )
- Ethylenediaminetetraacetic acid (EDTA) (Fisher BioReagents, Fisher Scientific, catalog number: BP120-500 )
- EDTA-free Protease Inhibitor Cocktail (Sigma-Aldrich, Roche Diagnostics, catalog number: 11873580001 )
- SYPRO Ruby protein gel stain (Bio-Rad Laboratories, catalog number: 1703125 )
- Bio-Safe Coomassie stain (Bio-Rad Laboratories, catalog number: 1610786 )
- Potassium sulfate (K2SO4) (Fisher Chemical, Fisher Scientific, catalog number: P304-3 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Fisher Chemical, Fisher Scientific, catalog number: C79-500 )
- Bacto dehydrated culture media additive: Tryptone (BD, BactoTM, catalog number: 211705 )
- BBL dehydrated culture media additive: Yeast extract (BD, BBLTM, catalog number: 211929 )
- Sodium hydroxide (NaOH) (Fisher Scientific, catalog number: BP359-212 )
- Agar (Sigma-Aldrich, catalog number: A7002 )
- Tris-base (Fisher BioReagents, Fisher Scientific, catalog number: BP152-1 )
- Concentrated HCl (Fisher Chemical, Fisher Scientific, catalog number: A481-212 )
- Sodium dodecyl sulfate (SDS) (Fisher BioReagents, Fisher Scientific, catalog number: BP166-500 )
- Bromophenol blue (Sigma-Aldrich, catalog number: B5525 )
- β-Mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- Acrylamide/bis-acrylamide (37.5:1, 40%) (electrophoresis grade) (Fisher BioReagents, Fisher Scientific, catalog number: BP1410-1 )
- Tetramethylethylenediamine (TEMED, electrophoresis grade) (Fisher BioReagents, Fisher Scientific, catalog number: BP150-100 )
- Ammonium persulfate (APS) (Bio-Rad Laboratories, catalog number: 1610700 )
- 2-(N-Morpholino)ethanesulfonic acid (MES) hydrate (ACROS Organics, catalog number: 172595000 )
- Methanol (Fisher Chemical, Fisher Scientific, catalog number: A413-4 )
- Tween 20 (molecular biology grade) (Sigma-Aldrich, catalog number: P9416 )
- Glycine (Bio-Rad Laboratories, catalog number: 1610718 )
- Amersham ECL prime Western blotting detection reagent (ECL Prime) (GE Healthcare, catalog number: RPN2232 )
- Precision plus protein dual color standards (500 µl) (Bio-Rad Laboratories, catalog number: 1610374 )
- ATCC974 medium (see Recipe 1)
- LB medium (see Recipe 2)
- Lysis buffer for HisTrap HP chromatography (see Recipe 3)
- Lysis buffer for StrepTrap HP chromatography (see Recipe 4)
- Tris-salt buffer (see Recipe 5)
- Concentrated assay buffer (see Recipe 6)
- 2x SDS reducing buffer (see Recipe 7)
- 12% SDS-PAGE gels (see Recipe 8)
- 10x running buffer (see Recipe 9)
- Transblot buffer (see Recipe 10)
- 10x TBS (see Recipe 11)
- Tris-buffered saline with 0.1% Tween 20 (TBST) (see Recipe 12)
Equipment
- Sorvall Evolution RC centrifuge (Thermo Fisher Scientific, Thermo ScientificTM, model: Sorvall Evolution RC, catalog number: 728211 ) (used to centrifuge samples at 4 to 15 °C and ≤ 12,000 x g)
- Fiberlite F9-4x 1000y fixed angle superspeed rotor (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 76981 ) and Sorvall SS-34 fixed angle rotor (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 28020 ) (used with Sorvall Evolution RC to centrifuge 1 L cultures and 50 ml cell extract, respectively)
- French Press G-M (Glen Mills, model: Model 11, catalog number: 5500-000011 ) (used for lysis of cells at 20,000 to 24,000 psi)
- French Press standard pressure cell (35 ml, 40,000 psi) (Glen Mills, catalog number: 6800-FA-032 ) (used with French Press, item 3)
- Sorvall RC-3 general purpose centrifuge (Newton, CT) (used for centrifugal filtration with an HL-8 swinging bucket rotor at 4 °C and 4,000 x g)
- Water bath (LAUDA-Brinkmann, model: LAUDA Aqualine AL 12 , catalog number: L000610) (used to incubate reactions at 45 °C)
- Two bench-top microcentrifuges (Eppendorf, model number: 5418 , catalog number: 022620304) (used for centrifugation of 1-2 ml samples at up to 10,000 x g) (place one centrifuge in the cold room for 4 °C and the other on the bench-top for room temperature)
- BenchRocker 2D rocker (Alkali Scientific, catalog number: RS7235 )
- Autoclave (Consolidated Sterilizer Systems, model: SR-24C )
- Mini-PROTEAN Tetra Handcast Systems (Bio-Rad Laboratories, catalog number: 1658005 )
- SmartSpec 3000 Plus spectrophotometer (Bio-Rad Laboratories, catalog number: 1702525 ) (use for BCA assay at A562 nm and monitoring of growth at OD600)
- PowerPac Basic 300 V power supply (Bio-Rad Laboratories, catalog number: 1645050 ) (used for transblot overnight at 20 V and SDS-PAGE for up to 90 min at 150 V)
- New Brunswick I24 shaker, 3/4" orbit (7-60 °C) (Eppendorf, New BrunswickTM, model: I24 , catalog number: M1344-0000) (used for culturing cells at 37 to 42 °C at 200 rpm)
- Refrigerator (4 °C) (Frigidare, model: FRU17B2JW )
- Puffer Hubbard (-20 °C) (Revco Tech, model: 1UF1821A14 )
- Ultra-low temperature freezer (-80 °C) (Eppendorf, New BrunswickTM, model: C660-86 )
- XP-Series toploading balance (Denver Instrument, model: XP-600 )
- Analytical balance (Mettler Toledo, model: B balance line, AB54 )
- Vortex mixer (Thermolyne, catalog number: 37600 )
- Chemical fume hood (St. Charles Manufacturing, St. Charles, IL)
- Pipettes (2-20 µl, 20-200 µl, 100-1,000 µl) (Rainin type LTS)
- pH meter (Corning, model: Model 320) (used to titrate buffers to pH 6.8 to 8.8)
- Scanner (Epson Perfection, model: 3170 Photo ) (used for scanning exposed X-ray film and Coomassie Blue R250 stained protein gels at 300-600 dpi)
- Mini trans-blot module (Bio-Rad Laboratories, catalog number: 1703935EDU )
- Konica X-ray film processor (Konishiroku Photo Industry, model: QX60A )
- Electrophoresis systems autoradiography cassette (Fisher Scientific, catalog number: FBXC-810 )
- Siemens Vantage Reverse Osmosis Systems (Siemens, model: M21 series, model number: M21R004EA ) with EVOQUA filters (Siemens, catalog number: C1207098 ) and Atlantic Ultraviolet Germicidal UV Equipment (Atlantic Ultraviolet, model: MP49 ) (use water purification system to generate deionized water)
- BioLogic DuoFlow 10 System (Bio-Rad Laboratories, catalog number: 7600037 ) (used for protein chromatography by step gradient at flow rates of 0.5 to 1.2 ml∙min-1 and monitoring of protein fractions by A280)
- Stirring hotplate (PC-220 Pyroceram) (Corning, catalog number: 6795-220 ) used with magnetic stir bars (Octagonal Magnetic Stir Bar Kit) (Fisherbrand, Fisher Scientific, catalog number: 14-513-82 )
Procedure
- Purify proteins for in vitro reconstitution assay
- Prepare the H. volcanii cells for MsrA-StrepII and His6-UbaA purification as follows.
- Using a sterile toothpick, streak H. volcanii LR03-pJAM3010 (MsrA-StrepII) and LR03-pJAM1209 (His6-UbaA) from -80 °C glycerol stocks onto agar plates of ATCC974 medium with novobiocin (0.2 μg∙ml-1) (ATCC+Nv medium, Recipe 1). The stocks consist of stationary phase cells frozen in ATCC+Nv medium with 15 % (v/v) glycerol.
- Incubate the plates in plastic zip-lock bags at 42 °C for 5 days until isolated colonies appear.
- Using a sterile loop, transfer the isolated colonies from the plates into 4 x 25 ml ATCC+Nv medium (in 250 ml baffled culture flasks covered in foil). Grow the cells at 42 °C with rotary shaking at 200 rpm to log-phase (OD600 of 0.6-0.8).
- Transfer the log phase Hfx. volcanii cells to fresh 4 x 1-L ATCC+Nv medium (in 2.8-L Fernbach flasks covered in foil). Grow the cells to stationary phase (OD600 of 3.5-4.0) at 42 °C with rotary shaking at 200 rpm.
Note: Ectopic expression of MsrA-StrepII and His6-UbaA are constitutive in the H. volcanii strains; hence, an inducer is not required.
- Using a sterile toothpick, streak H. volcanii LR03-pJAM3010 (MsrA-StrepII) and LR03-pJAM1209 (His6-UbaA) from -80 °C glycerol stocks onto agar plates of ATCC974 medium with novobiocin (0.2 μg∙ml-1) (ATCC+Nv medium, Recipe 1). The stocks consist of stationary phase cells frozen in ATCC+Nv medium with 15 % (v/v) glycerol.
- Prepare the E. coli cells for MsrA-StrepII and Flag-His6-SAMP2 purification as follows.
- Freshly transform plasmids pJAM3200 (for purification of MsrA-StrepII) and pJAM1132 (for purification of Flag-His6-SAMP2) into E. coli Rosetta (DE3) by chemical transformation as previously reported (Hanahan et al., 1991).
- Select for transformants by spread plating cells onto solid LB medium (Recipe 2) with kanamycin (50 μg∙ml-1) (LB+Km medium) and incubating the plates at 37 °C for 14 h.
- Transfer ~100 colonies of the freshly transformed cells into 25 ml LB+Km liquid medium (in 250 ml baffled culture flasks covered in foil) by adding 5 ml LB+Km medium to the plate using a 10 ml pipettor for resuspension and transfer.
Note: Not all proteins require this type of approach but we do find that a subset of the proteins we work with are not readily expressed in E. coli if the cells are not freshly transformed. Note that some proteins are expressed in E. coli using the pET system even in the absence of IPTG which can cause mild toxicity and selection for cells that display loss of target gene expression. We did not want to take this type of risk that the MsrA and/or other proteins of this study would be of low level expression in E. coli. - Grow the cells in the 25 ml medium at 37 °C with rotary shaking at 220 rpm to log phase (OD600 of 0.6-0.8).
- Subculture the cells into 500 ml fresh LB+Km medium (in 2.8 L Fernbach flasks covered in foil) to a final OD600 0.01 and grow the cells to log phase (OD600 of 0.6-0.8) at 37 °C with rotary shaking at 220 rpm.
- Induce protein expression in E. coli by addition of 0.4 mM IPTG. After induction, shift cell cultures to 25 °C for 12 h at 200 rpm prior to harvest.
- Freshly transform plasmids pJAM3200 (for purification of MsrA-StrepII) and pJAM1132 (for purification of Flag-His6-SAMP2) into E. coli Rosetta (DE3) by chemical transformation as previously reported (Hanahan et al., 1991).
- Harvest the H. volcanii and E. coli cells by centrifugation (4,500 x g, 10 min, 15 °C) using a Sorvall Evolution RC centrifuge with a Fiberlite F9-4x 1000y fixed angle superspeed rotor.
- Resuspend the cells at 4-5 vol per wet weight of cell pellet in lysis buffer formulated for HisTrap or StrepTrap HP chromatography (Recipes 3 and 4).
- Passage the resuspended cell material 4-6 times through a chilled French Press cell with a French Press at 24,000 psi. Collect the cell lysate in 50 ml polypropylene Nalgene centrifuge tubes on ice.
- Clarify the cell lysate by centrifugation (12,000 x g, 20 min, 4 °C) using a Sorvall Evolution RC centrifuge with a Sorvall SS-34 fixed angle rotor.
- Filter the cell lysate using SFCA syringe filters (0.8 μm then 0.45 µm pore size).
- Perform all chromatography steps at room temperature (as halophilic proteins are typically thermotolerant). Equilibrate the columns with 5-10 column volumes of Tris-salt buffer pH 7.4 (Recipe 5).
- Warm the clarified lysate to room temperature and apply the sample to a HisTrap HP column (5 ml bed volume, GE Healthcare) at a flow rate of 1 ml∙min-1 or StrepTrap HP column (1 ml bed volume, GE Healthcare) at a flow rate of 0.5 ml∙min-1. Control the flow rate of the chromatography using a BioLogic DuoFlow 10 System. Monitor protein fractions by A280.
- Remove the unbound proteins by washing the column with 140 ml Tris-salt buffer pH 7.4 at a flow rate of 1.2 ml∙min-1.
- Elute the bound proteins in Tris-salt buffer pH 7.4 supplemented with 5 mM d-desthiobiotin (for the StrepII tagged proteins) or 500 mM imidazole (for the His6-tagged proteins). Collect 1 ml fractions in 13 mm borosilicate glass tubes.
- Reducing 12% SDS-PAGE can be carried out using small portions of the eluted fractions (typically 5 μl is sufficient), followed by Coomassie Blue staining, to confirm the presence of the target protein in the eluted fractions.
- Concentrate the eluted protein fractions by centrifugal filtration using an Ultra-4 centrifugal filter unit with Ultracel-3 membrane (Merck) in an HL-8 swinging bucket rotor at 4 °C and 4,000 x g. We recommend concentrating the eluted fractions to a final volume of approximately 1 ml.
- Further purify the proteins by gel filtration chromatography by applying 0.5 ml of protein sample onto a Superdex 75 10/300 GL column (GE Healthcare) equilibrated in Tris-salt buffer pH 7.4 at a flow rate of 0.3 ml∙min-1. Collect peak fractions based on A280. Repeat this chromatographic step as needed. His6-UbaA purifies as a dimer, while Flag-His6-SAMP2 and MsrA-StrepII purify as monomers.
- Check the purity of eluted fractions as indicated in Step A12 above. The volume of each fraction applied to the gel should be adjusted based on the A280: typically for an A280 of 0.5, 7.5 μl of protein is sufficient.
- Repeat Steps A13- A15 as necessary until desired purity is achieved. Usually, two successive gel filtration chromatography steps will result in a highly purified preparation of protein.
- Once the protein preparation is of sufficient purity, concentrate the protein using an Amicon Ultra-4 to a final volume of 100 μl or less.
- Measure the protein concentration by BCA Protein Assay according to the supplier using bovine serum albumin (BSA) as the protein standard. Store the purified proteins at 4 °C for future assay.
Note: The purified proteins can be stored at 4 °C for 1 month.
- Prepare the H. volcanii cells for MsrA-StrepII and His6-UbaA purification as follows.
- Preparation of cell lysate for in vitro reconstitution assay
- Grow cultures of Hfx. volcanii LR03 (Δsamp1 Δsamp2 Δsamp3 ΔmsrA ΔubaA) in 25 ml of ATCC974 medium similarly to Steps A1a to A1c.
- Subculture cells to an OD600 of 0.01 in 500 ml ATCC974 medium with and without 25 mM DMSO (in 2.8 L Fernbach flasks). Grow cells to stationary phase (OD600 of 3.5-4.0) at 42 °C with rotary shaking at 200 rpm.
- Harvest cells by centrifugation (4,500 x g, 10 min at 15 °C) using a Sorvall Evolution RC centrifuge with a Fiberlite F8-6 x 1000y fixed angle superspeed rotor.
- Resuspend cells in 8 ml of Tris-salt buffer, pH 7.4, 10 mM MgCl2, 100 µM bortezomib (LC Laboratories), and 30 μg∙ml-1 DNase I from bovine pancreas (Sigma-Aldrich). Dilute the bortezomib from a 200 mM stock of bortezomib dissolved in 99.8% (wt/vol) DMF.
- Lyse cells by French Press (20,000 psi) (similarly to Step A5).
- Clarify the cell lysate by centrifugation (12,000 x g, 20 min, 4 °C) using a Sorvall Evolution RC centrifuge with a Sorvall SS-34 fixed angle rotor.
- Filter the lysate by using a 0.2 μm SFCA syringe filter.
- Study the effect of different chemical agents on the stimulation of SAMP conjugate formation by dialyzing (3 times, at 4 °C) the cell lysate against 50 mM Tris-Cl, pH 7.4 supplemented with 2 M NaCl. As an example, load 7 ml of cell lysate into the dialysis tubing (3.5 kDa MWCO, SnakeSkin pre-wet according to the manufacturer’s instructions, Fisher Scientific). Place the sample in 4 L of buffer and stir the buffer for 1.5 to 2 h at 4 °C. Replace the dialysis buffer with fresh buffer and dialyze the sample for an additional 1.5 to 2 h at 4 °C. Repeat this last step for a total of three buffer exchanges against the sample.
- Measure protein concentration of cell lysate by BCA Protein Assay (Thermo Scientific, Rockville, IL) according to the supplier using bovine serum albumin (BSA, Thermo Scientific) as the protein standard. Store cell lysate at 4 °C for future assay.
Note: We recommend using cell lysate for in vitro assay within 8 h after preparation.
- Grow cultures of Hfx. volcanii LR03 (Δsamp1 Δsamp2 Δsamp3 ΔmsrA ΔubaA) in 25 ml of ATCC974 medium similarly to Steps A1a to A1c.
- In vitro reconstitution of MsrA-dependent sampylation
- In a microcentrifuge tube, combine each component listed in the table below in the order shown.

Notes:- If the purpose is to purify and identify SAMP conjugates from the in vitro reconstitution assay, set the total reaction volume to 1.6 ml and use a 2.0 ml microcentrifuge tube.
- We always use freshly prepared concentrated assay buffer for every in vitro reconstitution assay. Mg-ATP, DMSO and protein components are selectively removed from the assay as controls. MsrA-StrepII can be purified from E. coli or H. volcanii LR03 (ΔmsrA ΔubaA Δsamp1/2/3, a sampylation deficient strain) with no detectable effect on conjugate formation.
- *Indicated molar concentration of His6-UbaA reflects the molecular mass of a His6-UbaA monomer, although His6-UbaA is purified as a dimer. For example, 5 µM* His6-UbaA is equivalent to 154 μg·ml-1 His6-UbaA.
- If the purpose is to purify and identify SAMP conjugates from the in vitro reconstitution assay, set the total reaction volume to 1.6 ml and use a 2.0 ml microcentrifuge tube.
- Incubate the reaction in a 45 °C water bath for 0, 2, 4, 8 and 18 h.
- Divide the 1.6 ml reaction sample into 2 parts (80 µl for Western blot and remaining 1.52 ml for purification of SAMP conjugates as described in Procedure D). After assay, remove salt from the 80 µl reaction mixtures using Zeba Spin Desalting Columns (7K MWCO) according to the supplier’s instructions (Thermo Scientific). Prior to application of the sample, equilibrate the column in 50 mM Tris-Cl buffer at pH 6.8. Centrifuge the column at 1,500 x g for 1 min using the Eppendorf microcentrifuge at room temperature. The final sample volume obtained is 100 µl.
- Terminate the reaction by mixing the desalted samples with an equal volume of 2x SDS reducing buffer (Recipe 7). Boil the samples for 3 x 5 min. Vortex for 30 sec after each boiling.
- Apply the reaction products to lanes 2-10 and the precision plus protein dual color molecular mass standards (2.5 μl) to lane 1 of reducing 12% SDS-PAGE gels (Recipe 8). The volume of sample applied may need to be adjusted based on detection sensitivity; good starting points are 3.5 µl for detection by anti-Flag antibody, and 6 µl for anti-StrepII or anti-His antibody. Separate the samples by electrophoresis at room temperature in 1x running buffer (diluted from 10x stock, Recipe 9) at 150 V for 70-90 min.
- Transfer the proteins to PVDF membrane at 4 °C using the mini trans-blot module in transblot buffer (Recipe 10) according to the supplier’s instructions (Bio-Rad) for 3 h at 90 V or overnight at 20 V.
- Remove the membrane from the cassette. Mark the location of the gel and the protein standards on the membrane using a pencil.
- Place the membrane upright in an 18 by 10 cm plastic container. Briefly rinse the membrane with 25 ml of 1x TBS.
- Block the membrane for 1 h at room temperature or overnight at 4 °C in 25 ml blocking buffer composed of TBST buffer (Recipe 11-12) supplemented with 10% (w/v) nonfat dry milk (Publix). Slowly rock the membrane during this blocking step on the medium setting of the BenchRocker 2D Rocker (Alkali Scientific Inc).
- Add the primary antibody to 10 ml of the blocking buffer in the following dilutions: 1:5,000 of the anti-Strep-tag II antibody to detect MsrA-StrepII, 1:5,000 of the anti-His IgG2 monoclonal antibody to detect His6-UbaA, and 1:10,000 of the alkaline phosphatase-linked anti-Flag M2 monoclonal antibody to detect Flag-His6-SAMP2. Incubate the membrane with the primary antibody solution for 60 min at room temperature using the medium setting of the rocker.
- Rinse the membrane for 3 x 15-30 min at room temperature with 25-50 ml TBST buffer using the high setting of the rocker.
- To detect the anti-Strep-tag II and anti-His primary antibodies, incubate the membrane with a 1:10,000 dilution of the secondary antibody [goat anti-mouse IgG (whole molecule)-alkaline phosphatase-linked antibody] in blocking buffer and rinse the membrane 3 x in TBST buffer as described above.
- Visualize the antibody: protein complexes on the PVDF membrane by chemiluminescence using CDP-Star (Thermo Fisher Scientific) and exposure to X-ray film (Thermo Fisher Scientific) according to the supplier’s guidelines.
Note: The HRP-conjugated His6 monoclonal antibody can be used in place of the discontinued anti-His IgG2 monoclonal antibody. HRP activity is detected using ECL Prime with X-ray film according to the user guidelines.
- In a microcentrifuge tube, combine each component listed in the table below in the order shown.
- Purification of SAMP-conjugates from the in vitro reconstitution assay
- Purify sampylated proteins from the in vitro reconstitution assay by use of Flag-His6-SAMP2 as the substrate and cOmplete His-tag resin (Roche) as the purification resin.
- Equilibrate 200 μl of the His-tag resin (Roche) in phosphate-buffered saline (PBS) (50 mM NaH2PO4, 300 mM NaCl, pH 8.0).
- Mix the sample (1.5 ml) after the in vitro assay with 8.5 ml PBS [5 mM EDTA, 5 mM DTT, 1 mg∙ml-1 EDTA-free protease inhibitor cocktail (Roche), 50 mM NaH2PO4, 300 mM NaCl, pH 8.0] prior to application to the His-tag resin (200 µl). To enhance the protein yield, apply the flow-through of the sample to resin for one more time.
Note: Total reaction volume is 1.6 ml. Use 0.1 ml to analyze SAMP conjugation reaction products by Western blot and the remaining 1.5 ml to purify SAMP conjugates. - Remove nonspecific proteins by washing the His-tag resin with 40 column volumes of PBS buffer.
- Elute bound proteins from the resin by addition of 80 μl of PBS supplemented with 200 mM imidazole.
- Transfer the resin with elution buffer into a new microcentrifuge tube and rotate for 20 min at 4 °C. Centrifuge the sample at 10,000 x g for 10 min using the Eppendorf microcentrifuge at 4 °C (in the cold room).
- Carefully remove 70 μl of the supernatant without touching the resin.
- Mix the supernatant with an equal volume of 2x SDS reducing buffer and boil 2 x 5 min with 30 sec vortex after each boiling.
- Separate proteins by 12% SDS-PAGE. Analyze the samples in parallel gels by immunoblotting and staining for total protein by SYPRO Ruby (Bio-Rad) followed by Bio-Safe Coomassie (Bio-Rad) as directed by the manufacturer. Excise the major band of SAMP-conjugates in gel slices for mass spectrometry analysis. Include gel slices of the negative control in this analysis.
*Note: Apply 10 μl of the sample for total protein staining and subsequent excision for mass spectrometry analysis. For immunoblotting analysis, apply 3.5 µl for detection by anti-Flag antibody, and 6 µl for anti-StrepII or anti-His antibody.
- Purify sampylated proteins from the in vitro reconstitution assay by use of Flag-His6-SAMP2 as the substrate and cOmplete His-tag resin (Roche) as the purification resin.
Data analysis
Systematically add different components in the vitro assay to study the sampylation process. Terminate reactions at different time points to determine the optimal reaction time. In vitro reconstitution of MsrA-dependent sampylation has been published in mBio (Fu et al., 2017). Figure 1 for the representative data is originally published as Figure 4A in our previous paper (Fu et al., 2017). The 50 kDa band corresponds to monosampylated UbaA (Figure 1, lane 12). MsrA-dependent SAMP conjugates distinct from automodified UbaA were observed when all the components were added in the presence of DMSO (Figure 1, lane 14 and 19).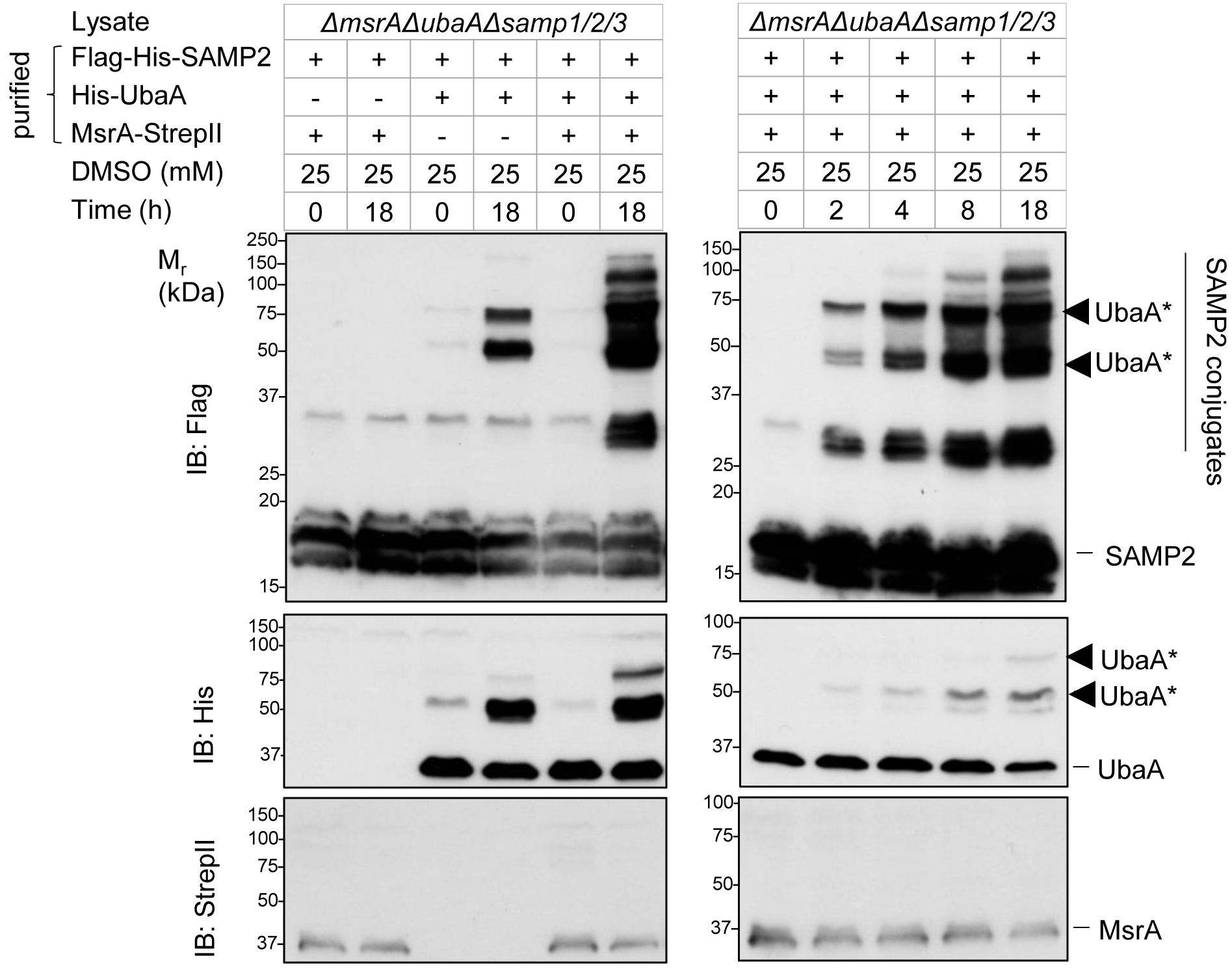
Figure 1. MsrA-dependent sampylation by in vitro reconstitution. Purified MsrA-StrepII was incubated with Flag-His6-SAMP2, His6-UbaA, and ATP (4 mM) for 0-18 h at 45 °C. DMSO was at a concentration of 25 mM. The reactions were supplemented with cell lysate of Hfx. volcanii LR03 (ΔmsrA ΔubaA Δsamp1/2/3, a sampylation deficient strain). Proteins after assay were separated by reducing 12% SDS-PAGE and analyzed by anti-StrepII, anti-Flag and anti-N-terminal His immunoblotting (IB) as indicated on the left. Migration of the molecular mass standards (Mr, kDa) is indicated on the left. Migration of SAMP2, SAMP2 conjugates, MsrA, UbaA, and sampylated UbaA (◄ UbaA*, the latter modified independent of MsrA) is noted on the right. The dataset of this figure was originally published in Fu et al. (2017).
Notes
Please note that our current understanding of sampylation in Archaea is incomplete. Thus, we use cell lysate of Hfx. volcanii LR03 (ΔmsrA ΔubaA Δsamp1/2/3, a sampylation deficient strain) as the resource for protein substrates and additional missing components required for sampylation. Future studies will be important to identify all the components required for sampylation and conduct complete in vitro reconstitution of sampylation using only the purified components of the system.
Recipes
- ATCC974 medium
- Dissolve 125 g NaCl, 50 g MgCl2·6H2O, 5 g K2SO4, 0.132 g CaCl2·2H2O, 5 g tryptone, and 5 g yeast extract in 750 ml deionized H2O. The medium will appear as a clear solution when all the chemicals are fully dissolved
- Adjust the pH of the solution to 6.8 by adding 0.5 N NaOH drop by drop
- Adjust the volume of the solution to final 1 L
- Autoclave on liquid cycle for 25 min
- Dissolve 125 g NaCl, 50 g MgCl2·6H2O, 5 g K2SO4, 0.132 g CaCl2·2H2O, 5 g tryptone, and 5 g yeast extract in 750 ml deionized H2O. The medium will appear as a clear solution when all the chemicals are fully dissolved
- LB medium
- Dissolve 10 g tryptone, 5 g yeast extract, and 10 g NaCl in 1 L deionized water
- Autoclave on liquid cycle for 25 min
- Dissolve 10 g tryptone, 5 g yeast extract, and 10 g NaCl in 1 L deionized water
Note: For Recipes 1 and 2, add antibiotics (as a powder) at the appropriate concentration to cooled media. For plates, add 15 g agar per L of media prior to autoclaving. After autoclaving, cool the media to 60 °C for 1-2 h and then dispense ~20 ml of the media per culture plate. Let the plates dry with lids on for 1-2 days at room temperature. Store liquid media in sterile bottles at room temperature or 4 °C in the dark (the latter if the media contains antibiotics or is a solid plate). Store plates and liquid cultures of H. volcanii at room temperature (not at 4 °C, as halophilic Archaea are sensitive to cold shock). Warm all media to room temperature prior to use.
- Lysis buffer for HisTrap HP affinity chromatography
50 mM Tris-Cl, pH 7.4
2 M NaCl
40 mM imidazole
1 mg∙ml-1 EDTA-free protease inhibitor cocktail (Roche) - Lysis buffer for StrepTrap HP chromatography
50 mM Tris-Cl, pH 7.4
2 M NaCl
1 mg∙ml-1 EDTA-free protease inhibitor cocktail (Roche) - Tris-salt buffer
50 mM Tris base
2 M NaCl
Titrated to pH 7.4 or 7.5 with concentrated HCl as indicated - Concentrated assay buffer
50 mM Tris-Cl, pH 7.5
40 mM ATP
250 mM DMSO
20 mM MgCl2
5 mM DTT
2 M NaCl - 2x SDS reducing buffer
100 mM Tris-Cl buffer at pH 6.8
4% (w/v) SDS
20% (v/v) glycerol
0.6 mg·ml-1 bromophenol blue
5% (v/v) β-mercaptoethanol - 12% SDS-PAGE gel
- Cast gel in Mini-PROTEAN Tetra Handcast System according to Bio-Rad with the following points of clarity
- Separating gel: 3.4 ml deionized H2O, 2.4 ml 40% acrylamide, 2 ml 1.5 M Tris pH 8.8, 80 μl 10% SDS, 80 μl 10% APS, and 8 μl TEMED
- Layer the top of the stacking gel with deionized H2O prior to the first polymerization
- Stacking gel: 2.9 ml deionized H2O, 0.75 ml 40% acrylamide, 1.25 ml 0.5 M Tris pH 6.8, 50 μl 10% SDS, 10% APS, and 5 μl TEMED
- The gel can be wrapped in wet Kimwipe and plastic wrap and stored at 4 °C for up to 1 week
- Cast gel in Mini-PROTEAN Tetra Handcast System according to Bio-Rad with the following points of clarity
- 10x running buffer
- Dissolve 30 g of Tris base, 144 g of glycine, and 10 g of SDS in 1 L of dH2O
- No pH adjustment is required. The pH of the buffer should be 8.3
- Store at room temperature and dilute to 1x before use
- Dissolve 30 g of Tris base, 144 g of glycine, and 10 g of SDS in 1 L of dH2O
- Transblot buffer
500 ml 0.2 M MES buffer pH 6
1 L 100% methanol
8.5 L deionized water
Store at 4 °C - 10x TBS (concentrated Tris-buffered saline)
- Dissolve 24 g Tris base and 88 g NaCl in 900 ml distilled H2O
- Adjust the pH to 7.6 with 12 N HCl
- Add distilled water to a final volume of 1 L
- Dissolve 24 g Tris base and 88 g NaCl in 900 ml distilled H2O
- Tris-buffered saline with 0.1% Tween 20 (TBST)
Mix 100 ml of TBS 10x with 900 ml distilled H2O and 1 ml Tween 20
Acknowledgments
This work was funded by US Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences, Physical Biosciences Program (DE-FG02-05ER15650), NIH | National Institute of General Medical Sciences (NIGMS) (NIH R01 GM57498-15) and USDA National Institute of Food and Agriculture (Hatch 1005900). This protocol was adapted from previous work published in Fu et al. (2017). The authors have no conflict of interest or competing interest to declare.
References
- Fu, X., Adams, Z., Liu, R., Hepowit, N. L., Wu, Y., Bowmann, C. F., Moskovitz, J. and Maupin-Furlow, J. A. (2017). Methionine sulfoxide reductase A (MsrA) and its function in ubiquitin-like protein modification in Archaea. MBio 8(5).
- Glickman, M. H. and Ciechanover, A. (2002). The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82(2): 373-428.
- Hanahan, D., Jessee, J. and Bloom, F. R. (1991). Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol 204: 63-113.
- Hepowit, N. L., de Vera, I. M., Cao, S., Fu, X., Wu, Y., Uthandi, S., Chavarria, N. E., Englert, M., Su, D., Sll, D., Kojetin, D. J. and Maupin-Furlow, J. A. (2016). Mechanistic insight into protein modification and sulfur mobilization activities of noncanonical E1 and associated ubiquitin-like proteins of Archaea. FEBS J 283(19): 3567-3586.
- Komander, D. and Rape, M. (2012). The ubiquitin code. Annu Rev Biochem 81: 203-229.
- Maupin-Furlow, J. A. (2014). Prokaryotic ubiquitin-like protein modification. Annu Rev Microbiol 68: 155-175.
- Zhao, Q., Liu, L. and Xie, Q. (2012). In vitro protein ubiquitination assay. Methods Mol Biol 876: 163-172.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Fu, X., Adams, Z. and Maupin-Furlow, J. A. (2018). In vitro Analysis of Ubiquitin-like Protein Modification in Archaea. Bio-protocol 8(10): e2845. DOI: 10.21769/BioProtoc.2845.
Category
Microbiology > Microbial biochemistry > Protein > Activity
Biochemistry > Protein > Activity
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










