- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Infection Process Observation of Magnaporthe oryzae on Barley Leaves
Published: Vol 8, Iss 9, May 5, 2018 DOI: 10.21769/BioProtoc.2833 Views: 9735
Reviewed by: Zhibing LaiJun YangWende Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Quantitative Estimation of Auxin, Siderophore, and Hydrogen Cyanide Production in Halo and Drought-Tolerant Bacterial Isolates for Cucumber Growth
Zeinab Fotoohiyan and Ali Salehi Sardoei
Oct 5, 2025 1342 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1568 Views

Reproducible Emu-Based Workflow for High-Fidelity Soil and Plant Microbiome Profiling on HPC Clusters
Henrique M. Dias [...] Christopher Graham
Jan 20, 2026 405 Views
Abstract
Rice blast and wheat blast caused by Magnaporthe oryzae is a serious threat to rice and wheat production. Appropriate methods for observing M. oryzae infection process are important for study of the fungal infection mechanisms, plant resistance reactions, and host-M. oryzae interactions. The rice leaf sheath is commonly used to inoculate M. oryzae for observing the infection process, however, this method is a time-consuming and high technical work. Here, we describe an easier solution to observe M. oryzae infection process on barley leaves.
Keywords: Magnaporthe oryzaeBackground
The filamentous fungus Magnaporthe oryzae can cause destructive rice blast and wheat blast diseases, which can also infect barley (Kohli et al., 2011; Dean et al., 2012). M. oryzae has been studied as a model to understand fungal-plant interactions (Yan and Talbot et al., 2016). This fungus initiates its infection by attaching the conidium to host surface, then the conidium germinates and forms a dome-shaped appressorium, by which it can penetrate into host cell for colonization (Wilson and Talbot, 2009). In host cells, the fungus colonizes as a biotrophic manner by forming bulbous and branched infection hyphae to interact with host defense system (Kankanala et al., 2007). M. oryzae sequentially invade living host cells and finally transformed into necrotrophic growth, during which the lesions appear and sporulation occurs. In order to study the fungal infection mechanism, or protein functions during infection, or plant defense reaction, it is required to observe cellular infection process of different strains in host cells. A rice leaf sheath method has been commonly used to observe the infection process (Koga et al., 2004), however, this method needs to waste a long time to prepare the rice leaf sheath, and the inoculation and sample preparation need a great deal of experience. Because barley is also the host of M. oryzae, and its leaf epidermis is easy for tearing, so we found that barley leaf epidermis method is an effective and simple method to observe the infection process of M. oryzae.
Materials and Reagents
- Petri dishes (6 cm and 9 cm diameters, ASONE)
- Lens paper (Fisher Scientific)
- Filter paper (Whatman)
- Medical gauze (Angyang Medical)
- Absorbent paper (Kimberly-Clark)
- 1.5 ml tubes (Eppendorf)
- Tips (10 μl, 200 μl and 1,000 μl, Axygen)
- Blades (Dexter Russell Cutlery, catalog number: 73-C )
- Glass slides (Fisher Scientific, catalog number: 12-550-343 )
- Coverslips (Fisher Scientific, catalog number: 12-547 )
- Pots (10 cm in diameter and 15 cm in height)
- Cultivated land soil
- Magnaporthe oryzae strains
Note: The M. oryzae strains are maintained on dried filter paper pieces stock in -80 °C for long-term storage. - Barley seeds (Hordeum vulgare, cv E9)
- Sterilized water (Milli-Q)
- Boiled oatmeal filtrate
- Tomato juice
- Agar (Sigma-Aldrich, catalog number: 17209 )
- Tween 20 (Sigma-Aldrich, catalog number: P9416 )
- Oatmeal tomato agar (OTA) solid media (see Recipes)
- 0.025% Tween 20 (see Recipes)
Equipment
- Scissor, tweezers, spreader
- Pipettes (Eppendorf, catalog numbers: 3124000016 [0.5-10 µl], 3124000032 [20-200 µl], and 3124000075 [100-1,000 µl])
- Incubation chamber (28 °C) for fungal growth and barley germination (Biolab Scientific, model: BIFG-101 )
- Hemocytometer (Marienfeld, catalog number: 0650030 )
- Greenhouse capable of temperature and humidity control for growing barley
- Optical microscope (Olympus, model: CX23 )
- Fluorescence microscope (Leica Microsystems, model: Leica DM2500 )
- Juice extractor
- Autoclave
Procedure
- Preparation of M. oryzae (Figure 1)
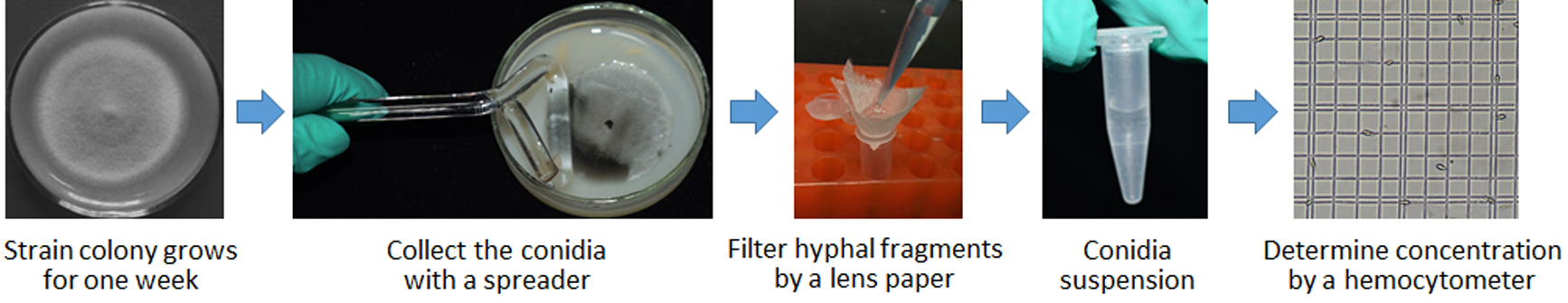
Figure 1. Preparation procedure for conidia suspension of M. oryzae- Inoculate the M. oryzae strains into solid OTA (Recipe 1) plates (6 cm diameter) and incubate for one week in an illumination incubator at 28 °C.
- Add 2 ml sterile distilled water containing 0.025% Tween 20 (Recipe 2) to each OTA plate and scrape with a spreader to harvest conidia. Use two layers of sterile lens paper to filter and remove hyphal fragments. Upon filtering, brief vortex by pipetting to re-suspend conidia.
Note: Tween 20 will help to increase water hydrophobic property, therefore promote the formation of the water drops without collapse. - Determine the conidia concentration by using a hemocytometer under an optical microscope, and adjust the final concentration to 1 x 105 per ml of 0.025% Tween 20 sterile water.
- Inoculate the M. oryzae strains into solid OTA (Recipe 1) plates (6 cm diameter) and incubate for one week in an illumination incubator at 28 °C.
- Preparation of barley leaves
- Put barley seeds (around 50 seeds) into a Petri dish with an appropriate volume of sterile water and place the Petri dish in an incubation chamber at 28 °C for 12 h.
- Remove the water, and wash the seeds for several times with sterile water, then hung up in one layer gauze to dry for 6 h.
- Put the seed into the Petri dish (9 cm diameter), and immerse the seeds with an appropriate volume of sterile water, then cover several layers of wet gauze. Place the Petri dish in an incubation chamber for 12-18 h at 28 °C.
- After the seeds germinate, transfer them into pots (10 cm in diameter and 15 cm in height) containing 80% volume of cultivated land soil (20-30 seeds per pot). Place the pots in a greenhouse for growth at room temperature. Water the pot every two days to keep the soil wet. After one week, the barley leaves can be used for inoculation (Figure 2).

Figure 2. Procedure for inoculation of M. oryzae on barley leaves
- Put barley seeds (around 50 seeds) into a Petri dish with an appropriate volume of sterile water and place the Petri dish in an incubation chamber at 28 °C for 12 h.
- Inoculation of barley with M. oryzae (Figure 2)
- Cut the barley leaves from the base with a scissor, then put them (back side up) in a Petri dish (9 cm diameter), which have been covered with two layers of water immersed absorbent paper. Fix the leaf base and tip with water immersed absorbent paper, and keep leaf flat (Video 1).
Note: When fix the leaves base and tip with water immersed absorbent paper, keep the leaves dry and don’t touch the leaves with fingers or any other materials.Video 1. Put barley leaves into the Petri dish and fix with water immersed absorbent paper - Drop the conidia suspension onto barley leaf beside the vein using a pipette (0.5-10 μl range) (Video 2).
Note: Keep each drop with a diameter of around 2-3 μm and without collapse, if the drop is too large or collapsed, it may tend to the formation of aerial mycelium, but not appressorium. Keep the tips away from the epidermis could avoid collapse. Re-suspending conidia by shaking up and down of the tubes every time before absorbing the conidia suspension.Video 2. Drop conidia suspension onto the barley leaves - Put on the lid, cover the Petri dishes with wetted absorbent papers, and place them into a sealed moist box. Then put the box in a dark incubator at 28 °C.
- Cut the barley leaves from the base with a scissor, then put them (back side up) in a Petri dish (9 cm diameter), which have been covered with two layers of water immersed absorbent paper. Fix the leaf base and tip with water immersed absorbent paper, and keep leaf flat (Video 1).
- Infection process observation (Figure 3)
- Takeout the barley leaves with a tweezer at different time points after inoculation. Use a blade to cross cut the leaf from up to down, at the tip side or base side. Keep the lower epidermis still connected, and subsequently tear down the lower epidermis (Figure 3A, Video 3).
Note: During this process, try best not to touch the water drops, which could break the infection structures. Recommend observing penetration pegs and primary infection hyphae at 18 hpi, and secondary infection hyphae at 24-30 hpi.Video 3. Tear down the barley lower epidermis and put onto a glass slide - Put the lower epidermis onto a glass slide, add some water to immerse the epidermis, then cover it with a coverslip.
- Observe the infection processes of M. oryzae in the cells of epidermis under a microscope, take images and calculate formation ratios of different infection structures (Figure 3A). For each time, more than 50 conidia should be calculated.
- Observe the subcellular localization of GFP-tagged proteins in infection structures under a fluorescence microscope (Figure 3B).
Note: During observing, remember to add water when the sample is dried.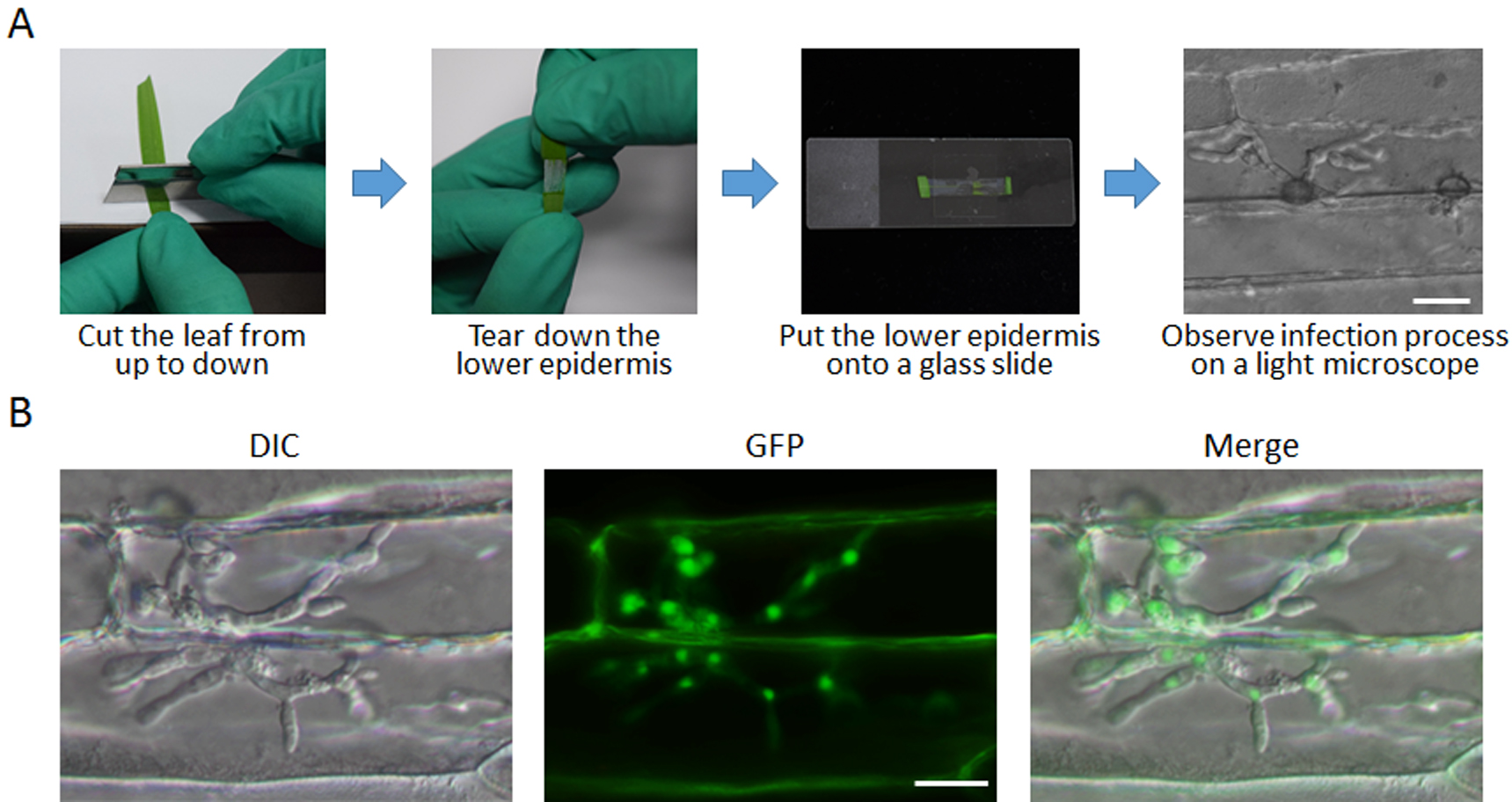
Figure 3. Procedure for observing of different samples. A. Procedure of observing the infection process. B. Observation of protein subcellular localization. DIC, differential interference contrast; GFP, green fluorescent protein. Scale bars = 20 μm.
- Takeout the barley leaves with a tweezer at different time points after inoculation. Use a blade to cross cut the leaf from up to down, at the tip side or base side. Keep the lower epidermis still connected, and subsequently tear down the lower epidermis (Figure 3A, Video 3).
Data analysis
All information about data processing, statistical tests, replicates and independent experiments were already included in the original research paper (Chen et al., [2014], N-glycosylation of effector proteins by an α-1,3-mannosyltransferase is required for the rice blast fungus to evade host innate immunity. Plant Cell 26(3):1360-1376. doi: 10.1105/tpc.114.123588).
Notes
- Plant healthy is very important for the success of the infection assay. Barley plant should grow under adequate sunshine, and avoid soil drought. Otherwise, the barley epidermis can’t be well infected with M. oryzae.
- Accurately addition of 0.025% Tween 20 into the sterile water is important for successful inoculation. The tips should be cut off before absorbing the reagent. Absorb the Tween 20 slowly and the adhering reagent on tips should be removed.
- To prevent the drops from collapse during inoculation steps, the Petri dish should be moved as slowly as possible.
Recipes
- Oatmeal Tomato Agar (OTA) solid media (1 L)
40 g boiled oatmeal filtrate
150 ml tomato juice
20 g agar- Boil the oatmeal in 800 ml distilled water for 30 min, then filtrate the mixture by double-layer gauze
- Extract tomato juice by an extractor, then also filtrate the mixture by double gauze. Mix 150 ml tomato juices with boiled oatmeal filtrate, then add water to 1 L
- Add 20 g agar, and autoclave for 40 min
- Boil the oatmeal in 800 ml distilled water for 30 min, then filtrate the mixture by double-layer gauze
- 0.025% Tween 20
Add 250 μl Tween 20 in 800 ml sterilized distilled water and adjust the volume to 1 L
Acknowledgments
The protocol was adapted from Chen et al. (2014). This work was supported by grants from the National Natural Science Foundation of China (31571952, 31601585). The author declares no conflict of interests.
References
- Chen, X. L., Shi, T., Yang, J., Shi, W., Gao, X., Chen, D., Xu, X., Xu, J. R., Talbot, N. J. and Peng, Y. L. (2014). N-glycosylation of effector proteins by an alpha-1,3-mannosyltransferase is required for the rice blast fungus to evade host innate immunity. Plant Cell 26(3): 1360-1376.
- Dean, R., Van Kan, J. A., Pretorius, Z. A., Hammond-Kosack, K. E., Di Pietro, A., Spanu, P. D., Rudd, J. J., Dickman, M., Kahmann, R., Ellis, J. and Foster, G. D. (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13(4): 414-430.
- Kankanala, P., Czymmek, K. and Valent, B. (2007). Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 19(2): 706-724.
- Koga, H., Dohi, K., Nakayachia, O. and Moria, M (2004). A novel inoculation method of Magnaporthe grisea for cytological observation of the infection process using intact leaf sheaths of rice plants. Physiol Mol Plant Pathol 64: 67-72.
- Kohli, M. M, Mehta, Y. R., Guzman, E, De Viedma, L. and Cubilla, L. E. (2011). Pyricularia blast – a threat to wheat cultivation. Czech J Genet Plant Breed 47:130-134.
- Wilson, R. A. and Talbot, N. J. (2009). Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 7(3): 185-195.
- Yan, X. and Talbot, N. J. (2016). Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr Opin Microbiol 34: 147-153.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Chen, X. (2018). Infection Process Observation of Magnaporthe oryzae on Barley Leaves. Bio-protocol 8(9): e2833. DOI: 10.21769/BioProtoc.2833.
Category
Microbiology > Microbe-host interactions > Fungus
Plant Science > Plant immunity > Host-microbe interactions
Cell Biology > Cell-based analysis > Fungal infection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











