- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantifying Podocytes and Parietal Epithelial Cells in Human Urine Using Liquid-based Cytology and WT1 Immunoenzyme Staining
Published: Vol 8, Iss 9, May 5, 2018 DOI: 10.21769/BioProtoc.2827 Views: 7327
Reviewed by: Di WangHaixia XuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

High-throughput Assessment of Mitochondrial Protein Synthesis in Mammalian Cells Using Mito-FUNCAT FACS
Hironori Saito [...] Shintaro Iwasaki
Feb 5, 2023 1867 Views

Immunofluorescent Staining Assay of 3D Cell Culture of Colonoids Isolated from Mice Colon
Trisha Mehrotra [...] Didier Merlin
Mar 5, 2024 2485 Views

Intraepidermal Nerve Fiber Quantification of the Mouse Hind Paw Footpads: A Detailed and Simplified Protocol
Anastasia Yerushkin [...] Amir Dori
Dec 5, 2025 1224 Views
Abstract
In glomerular disease, podocytes and parietal epithelial cells (PECs) are shed in the urine. Therefore, urinary podocytes and PECs are noninvasive biomarkers of glomerular disease. The purpose of this protocol is to employ immunocytochemistry to detect podocytes and PECs, using the WT1 antibody on liquid-based cytology slides.
Keywords: PodocytesBackground
Podocytes line the exterior of glomerular capillaries and thus face the Bowman’s capsule and primary urine. In glomerular injury, podocytes may detach from the glomerular basement membrane. The detachment of podocytes and their shedding in the urine have been implicated in the progression of glomerular diseases and crescent formation. Parietal epithelial cells (PECs) cover the inner aspect of the Bowman’s capsule. Similar to podocytes, it has been reported that PECs positive for WT1, proliferate and are shed in the urine during active glomerular disease (Zhang et al., 2012; Fujita et al., 2017).
Previous studies have shown a relationship between the number of urinary podocytes and PECs and various types of glomerular disease (Hara et al., 1998; Nakamura et al., 2000; Achenbach et al., 2008). The studies cited above used conventional methods (direct smears and cytospin) to prepare the urine samples. However, these conventional methods have common problems, including an air-drying effect and the possibility of significant cell loss during the cytocentrifugation and fixation processes. In addition, standardization and quantitative evaluation are difficult with the conventional methods. Although most previous studies have used immunofluorescence staining with the podocalyxin antibody, this method is difficult to perform in small- and medium-sized hospitals because of the specialty antigen and the requirement for a fluorescence microscope.
A useful urinary biomarker test should be easy to perform and have minimal requirements for standardized sample preparation and immunocytochemistry. Therefore, we have devised a method for detecting podocytes and PECs by combining liquid-based cytology, the WT1 antibody, and immunoenzyme staining. SurePathTM liquid-based cytology was developed as a replacement for the conventional sample preparation method because of its better cell preservation and higher cell recovery rate. SurePathTM allows the possibility of standardization and quantitative evaluation, and this system can be operated manually without machines. Furthermore, immunoenzyme staining using the WT1 antibody is performed in most pathological laboratories. Therefore, our method can be an inexpensive, simple, and internationally standardized method for the detection of urinary podocytes and PECs.
Materials and Reagents
- Saline-soaked gauze balls
- Medicine spoon
- Conical centrifuge tube (Corning, Falcon®, catalog number: 352097 ) or equivalent
- Kimwipes (KCWW, Kimberly-Clark, catalog number: 34133 ) or equivalent
- PAP pen (Abcam, catalog number: ab2601 ) or equivalent
- BD TotalysTM Slide Prep Slide Rack (BD, catalog number: 491294 )
- Coverslip (Matsunami Glass, catalog number: C024321 ) or equivalent
- BD SurePathTM Manual Method Kit (including settling chamber and positively charged slide) (BD, catalog number: 491266 )
- BD CytoRichTM Red Preservative Fluid (BD, catalog number: 491336 )
- Ethanol (Wako Pure Chemical Industries, catalog number: 059-06957 ) or equivalent
- Methanol (Wako Pure Chemical Industries, catalog number: 136-09475 ) or equivalent
- Hydrogen peroxide (Wako Pure Chemical Industries, catalog number: 086-07445 ) or equivalent
- Phosphate-buffered saline (PBS) (Nichirei Biosciences, catalog number: 415223 ) or equivalent
- WT1 antibody (clone 6F-H2) (Agilent Technologies, catalog number: M3561 )
- Antibody diluent (Agilent Technologies, catalog number: S3022 ) or equivalent
- N-Histofine® Simple StainTM MAX PO (MULTI) (Nichirei Biosciences, catalog number: 414151F )
- N-Histofine® DAB-3S Kit (Nichirei Biosciences, catalog number: 415192F )
- Mayer’s hematoxylin (Muto Pure Chemicals, catalog number: 30002 ) or equivalent
- Xylene (Wako Pure Chemical Industries, catalog number: 245-00717 ) or equivalent
- Malinol (mounting medium) (Muto Pure Chemicals, catalog number: 20093 ) or equivalent
Equipment
- Pipette (Nichiryo, model: Nichipet EXII ) or equivalent
- Centrifuge (Kubota, model: M4000 ) or equivalent
- Vortex mixer (Scientific Industries, model: Vortex-Genie 2 ) or equivalent
- Microscope (Olympus, model: BX43 ) or equivalent
Procedure
- Urine collection
- First-morning mid-stream-voided urine specimens are the most suitable for our method.
- Although spot mid-stream-voided urine is also likely usable, we have not verified such evidence.
- In catheter urine, WT1-positive cells are numerically diluted by mechanically detached urothelial cells.
- In the case of female samples, prior cleaning of the urethral meatus using saline soaked gauze balls before urine collection is preferable to prevent contamination by cells from the vulva. Additionally, samples collected during menstruation are not suitable for our method.
- First-morning mid-stream-voided urine specimens are the most suitable for our method.
- Cytology specimen preparation (SurePathTM liquid-based cytology)
The principle underlying the SurePathTM system is gravity sedimentation and electrical adhesion. In brief, the settling chamber is clamped to a positively charged slide, and the cell suspension is added. Gravity then settles the cells onto the slide, and the positively charged slide is combined with negatively charged cells (Figures 1 and 2).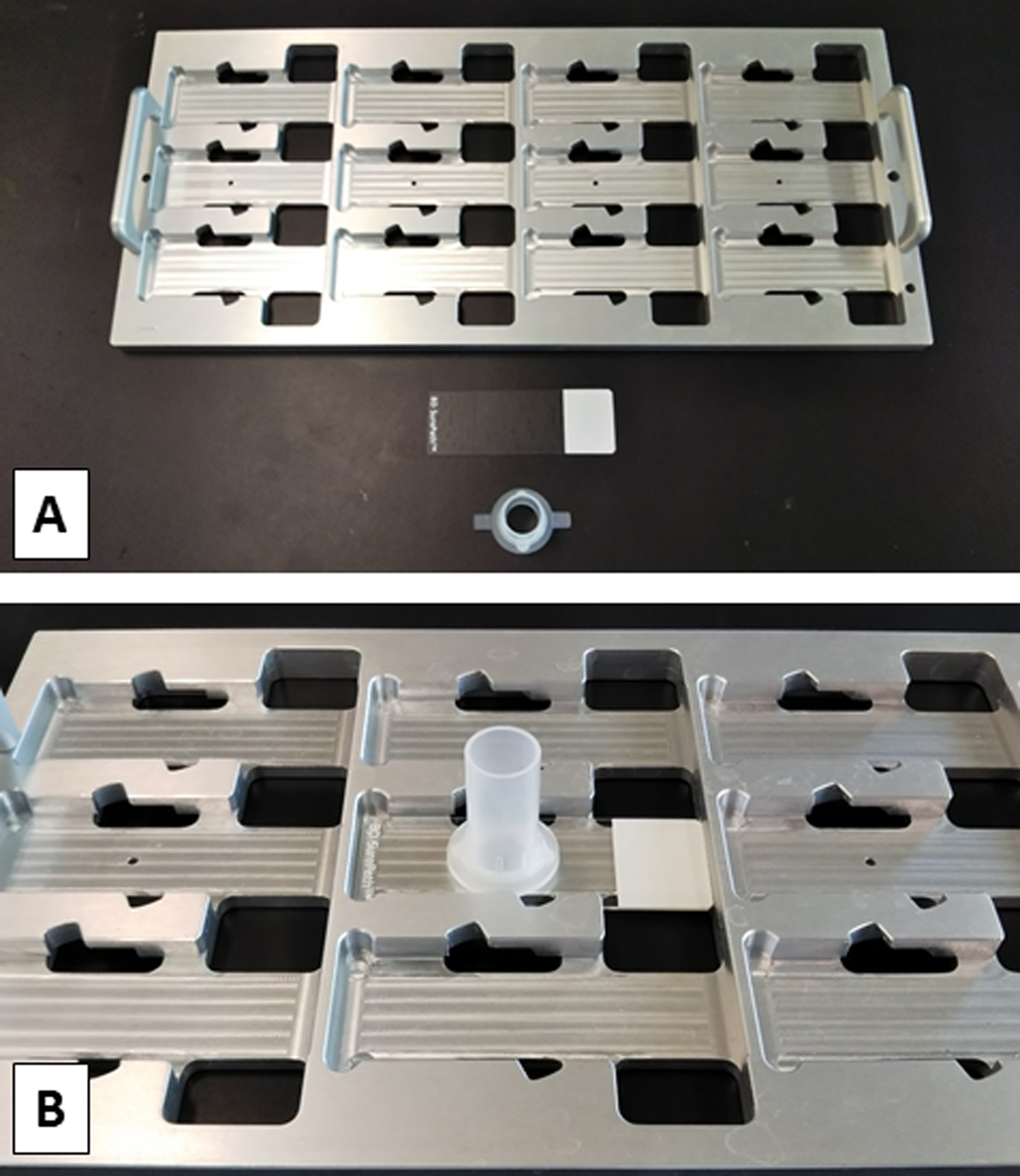
Figure 1. SurePathTM system manual method kit. A. Slide rack and manual method kit. B. A positively charged slide and a settling chamber set in a rack.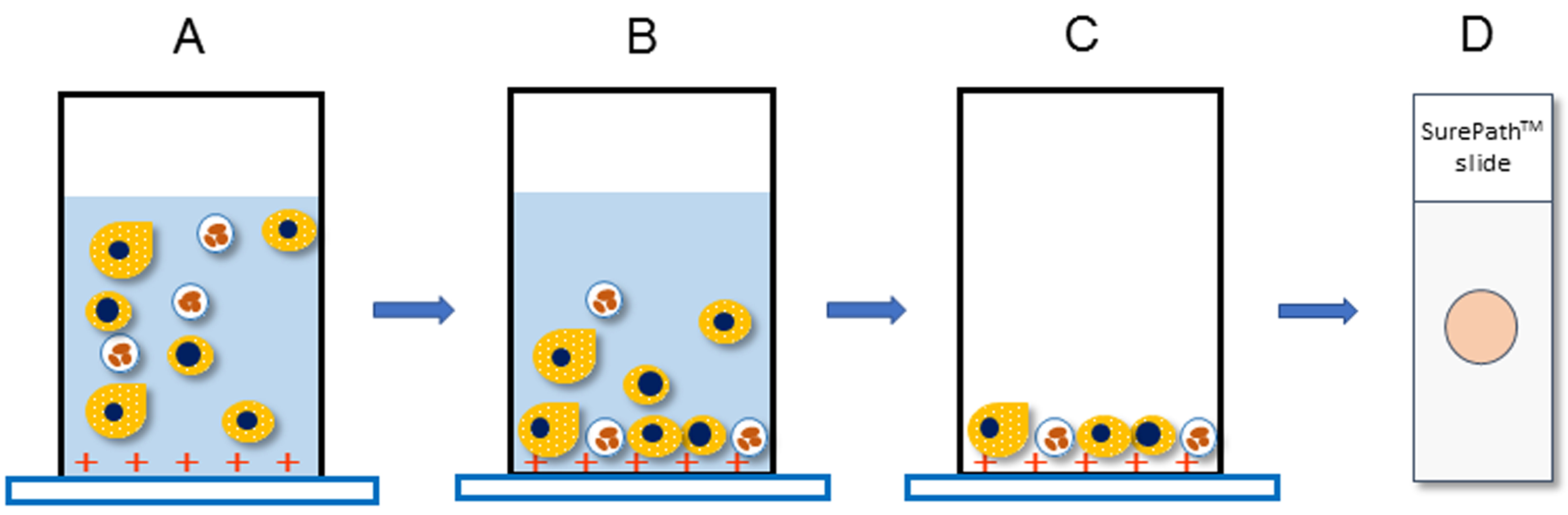
Figure 2. The principle underlying the SurePathTM system. A. Transfer the specimen to a settling chamber and mount on a positively charged slide. B. Negatively charged cells sediment by gravity and electrically combine with positively charged slides. C. Excess cells are removed together with the supernatant. D. SurePathTM slides consist of a 13 mm circular monolayer smear.- Stir the whole amount of urine with a medicine spoon to make it homogeneous.
- Distribute 10 ml of the homogeneous urine into a none-sterilized 15 ml conical test tube.
- Spin at 800 x g for 5 min in a swing-type centrifuge.
Note: The centrifuge should be swing-type, not angle-type. - Decant the supernatant with an aspirator or 5 ml pipette.
- Resuspend the sediment in 10 ml of CytoRich RedTM.
Notes:- In the case of macroscopic hematuria, collect and fix the buffy coat only after removal of the supernatant.
- The urine sediment needs to be fixed by CytoRich RedTM within 3 h after urine collection.
- Cells are stable for 3 days in CytoRich RedTM, they should not be stored longer than that.
- In the case of macroscopic hematuria, collect and fix the buffy coat only after removal of the supernatant.
- Fix the sample for at least 30 min.
- Centrifuge the specimens at 800 x g for 5 min and remove the supernatant.
- Add 6 ml of distilled water to the sediment and resuspend it for 10 sec with a vortex mixer.
- Centrifuge the specimen at 800 x g for 5 min and remove the supernatant.
- Add another 300 μl of distilled water to the sediment and resuspend it for 10 sec with a vortex mixer.
- After resuspension, transfer the entire amount of the specimen to a settling chamber and mount on a slide for 10 min.
- Invert the slide rack and discard the supernatant.
- Rinse the inside of the settling chamber with 95% ethanol.
Note: Do not apply 95% ethanol directly to smears (Figure 3). - Invert the slide rack and discard the 95% ethanol.
Note: Take care not to dry the smear. - Repeat once more Steps B13 and B14.
- Remove the settling chamber and immediately soak the slide in 95% ethanol.
Notes:- Immunostaining is carried out after fixation with 95% ethanol for 30 min or more.
- Cells are stable for 3 days in the 95% ethanol, they should not be stored longer than that.
- Immunostaining is carried out after fixation with 95% ethanol for 30 min or more.
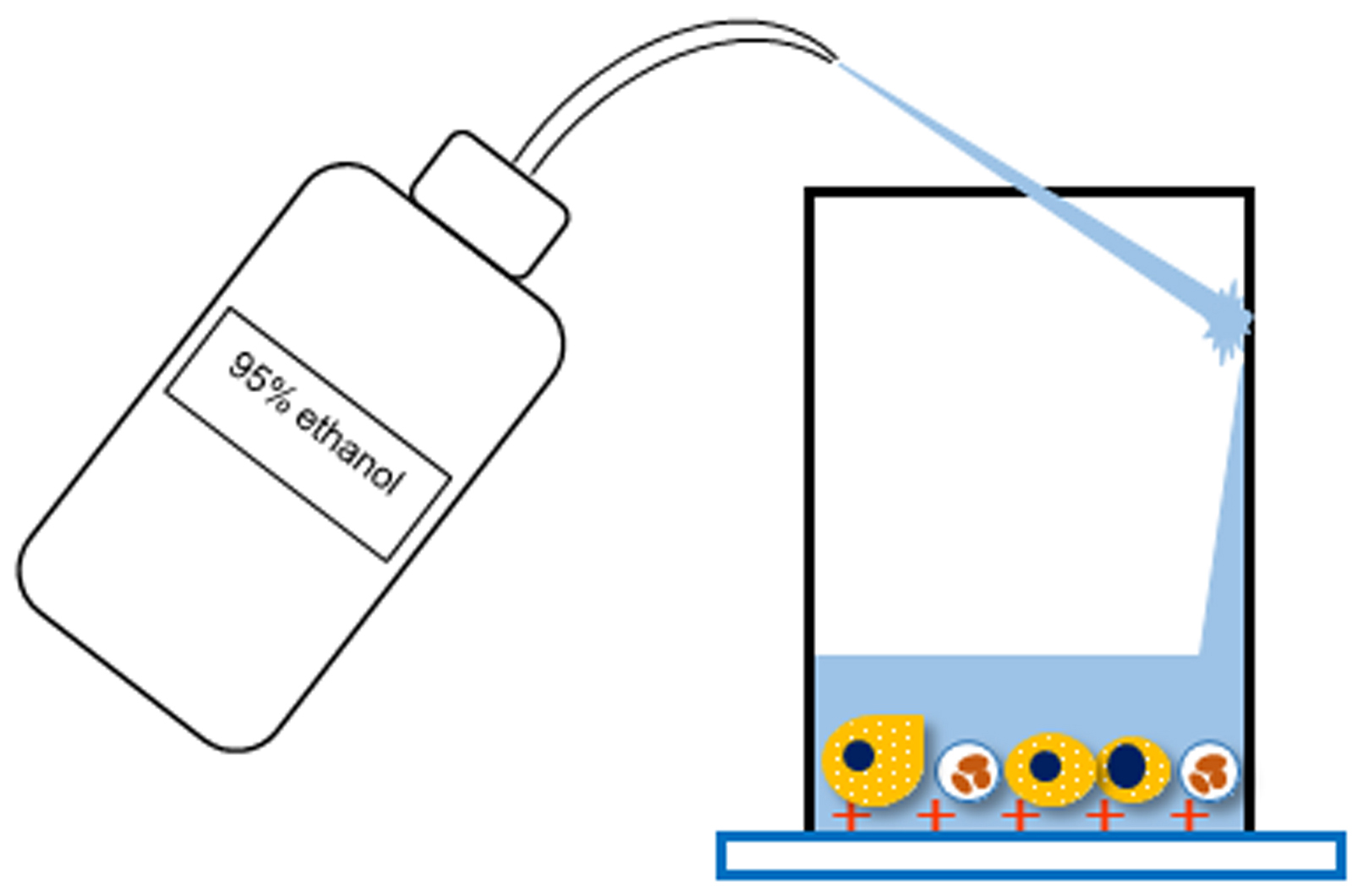
Figure 3. Schematic diagram of Step B13 - Stir the whole amount of urine with a medicine spoon to make it homogeneous.
- Immunocytochemistry (immunoenzyme staining)
- After fixing with 95% ethanol, incubate the slides in methanol containing 0.3% hydrogen peroxide to block endogenous enzyme activity (for 15 min).
Notes:- Hydrogen peroxide solution needs to be prepared freshly each time.
- In any process, when moving the slides through the solutions, mix the solutions and slides adequately by dipping with the slide holder several times.
- Hydrogen peroxide solution needs to be prepared freshly each time.
- Hydrate the slides in a series of ethanol solutions of decreasing concentration (70% ethanol, 50% ethanol, and distilled water, for 1 min each).
- Remove the slide and use Kimwipes to wipe away the distilled water surrounding the cell smear.
- Enclose the area around the smear with a PAP pen.
Notes:- Use the PAP pen on the part wiped dry of the distilled water.
- Move to the next step only after the PAP pen line has dried (for 10-20 sec).
- Use the PAP pen on the part wiped dry of the distilled water.
- Rinse the slide with PBS (2 times, for 1 min each time).
Note: If stored at 4 °C, PBS can be used for several months. - Incubate the cell smear with 100 µl of diluted (1:1,000) WT1 antibody in a humidified chamber (for 1 h at room temperature).
- Rinse the slide with PBS (3 times, for 1 min each time).
- Incubate the cell smear with 3 drops of N-Histofine Simple Stain MAX PO (MULTI) in a humidified chamber (for 30 min at room temperature).
- Rinse the slide with PBS (3 times, for 1 min each time).
- Incubate the cell smear with 100 µl of DAB reagent (for 1 min).
Note: Prepare the DAB reagent immediately before use. - Rinse the slide with distilled water (3 times, for 1 min each time).
- Counterstain the slide with Mayer’s hematoxylin (for 30 sec).
- Rinse the slide under running tap water (for 5 min).
- Dehydrate the slide with 100% ethanol (4 times, for 5 min each time).
- Move the slide from the ethanol into the xylene (4 times, for 5 min each time).
- Mount the coverslip onto the slide using mounting medium.
- After fixing with 95% ethanol, incubate the slides in methanol containing 0.3% hydrogen peroxide to block endogenous enzyme activity (for 15 min).
Data analysis
Count the WT1-positive cell numbers in a total field of smear under a light microscope (objective 20x or 40x).
Representative data:
- Urinary WT1-positive cells
Urinary WT1-positive cells exhibited moderate to strongly positive staining of the cytoplasm and were negative for the nuclei. The morphology of these cells was observed to be round to polygon-shaped, with a diameter of 20-50 μm and a clear or vacuolated cytoplasm (Figures 4A). The WT1-positive cells showed a variety of morphologies, ranging from anucleated to multinucleated cells. Occasionally, cast encasement of WT1-positive cells was present (Figures 4B) (Ohsaki et al., 2016).
Previous study has reported that WT1 exist not only at the nucleus, but also at cytoplasm (Niksic et al., 2004). The reason why urinary WT1-positive cells showed positive staining of the cytoplasm and were negative for nuclear staining in our method is that we employed an alcohol-based fixative (CytoRichTM Red). Since alcohol-based fixatives yield less reproducible staining for antibodies to nuclear epitopes (Gong et al., 2004), nuclear expression of WT1 is only detectable in formalin-fixed material (Skoog and Tani, 2011).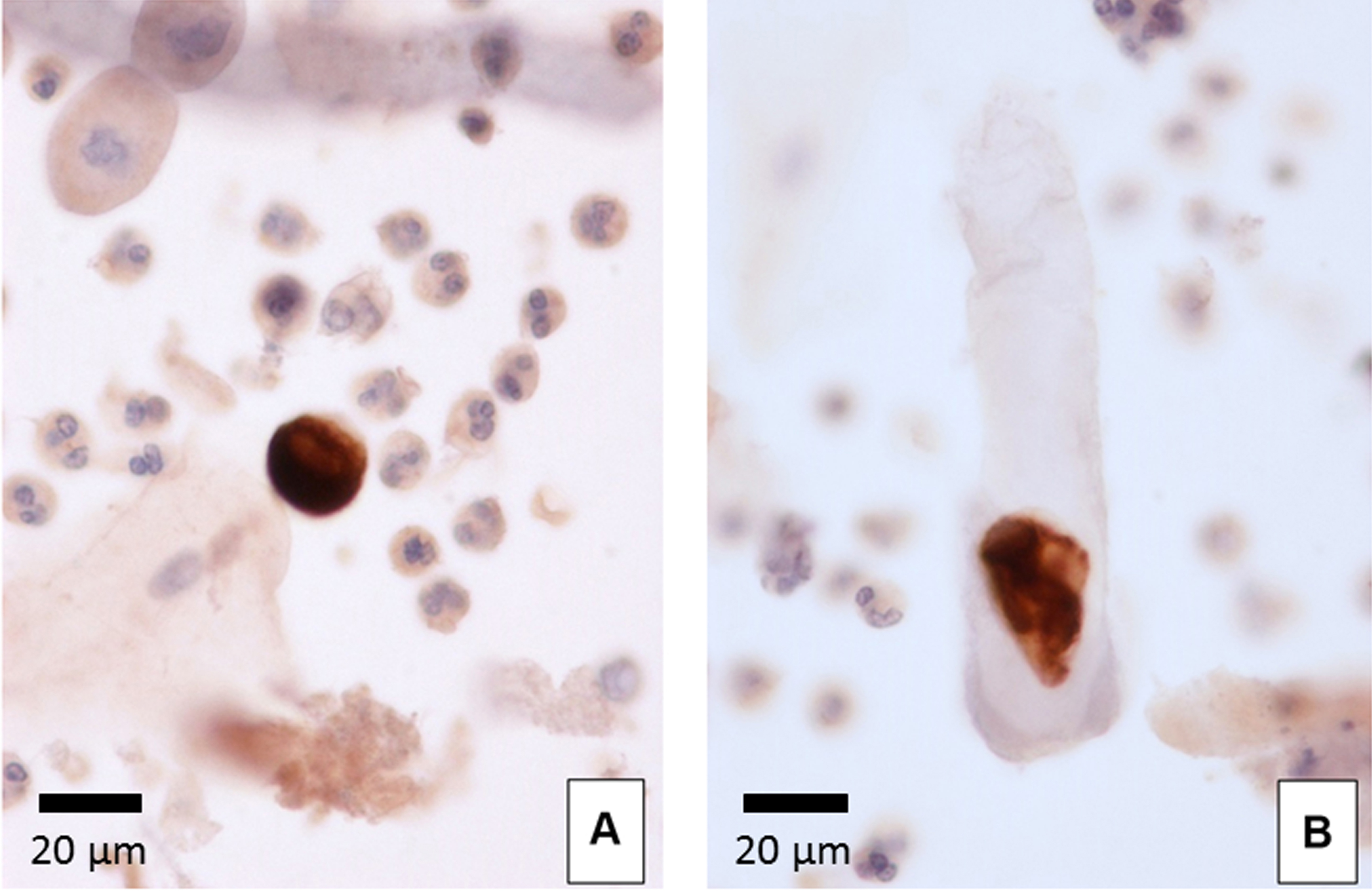
Figure 4. Urinary WT1-positive cells. WT1 immunocytochemistry (400x). A. WT1-positive cell surrounded by neutrophils and squamous cells. B. Cast encasement of a WT1-positive cell. - Correlation between urinary WT1-positive cells and glomerular disease
In patients with glomerular disease, WT1-positive cells were found in 33 samples (50.0%, 33/66). No WT1-positive cells were found in patients with benign lower urinary tract disease (0%, 0/45) and in healthy volunteers (0%, 0/30). The frequency of WT1-positive cells in glomerular disease was significantly higher than that in benign lower urinary tract disease (P < 0.001) and the healthy volunteer samples (P < 0.001) (Ohsaki et al., 2016). - Correlation between urinary WT1-positive cells and crescent formation
The number of urinary WT1-positive cells in patients with crescent formation was significantly higher than that in patients without crescent formation (P = 0.007) (Fujita et al., 2017). - Cutoff value of urinary WT1-positive cells
The best cutoff value of urinary WT1-positive cells to differentiate patients with crescentic lesion from those without the lesion was 5 cells/10 ml (sensitivity 73.3%, specificity 64.9%). Urinary WT1-positive cells produced an AUC of 0.735 (Fujita et al., 2017).
Recipes
- PBS
9.55 g of PBS powder
Bring the volume to 1,000 ml with distilled water - 0.3% hydrogen peroxide
1 ml 30% hydrogen peroxide
Bring the volume to 99 ml with methanol - WT1 antibody
1 μl WT1 antibody
Bring the volume to 999 μl with antibody diluent
Acknowledgments
Dr. Ohsaki was supported by a grant from The Kidney Foundation, Japan (Grant number: JKF12-3) and JSPS KAKENHI (Grant number: JP15K08382). The authors declare no conflict of interest.
References
- Achenbach, J., Mengel, M., Tossidou, I., Peters, I., Park, J. K., Haubitz, M., Ehrich, J. H., Haller, H. and Schiffer, M. (2008). Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol Dial Transplant 23(10): 3138-3145.
- Fujita, T., Sofue, T., Moritoki, M., Nishijima, Y., Tokuhara, Y., Wakisaka, H., Kushida, Y., Haba, R. and Ohsaki, H. (2017). Urinary WT1-positive cells as a non-invasive biomarker of crescent formation. Cytopathology 28(6): 524-530.
- Gong, Y., Symmans, W. F., Krishnamurthy, S., Patel, S. and Sneige, N. (2004). Optimal fixation conditions for immunocytochemical analysis of estrogen receptor in cytologic specimens of breast carcinoma. Cancer 102(1): 34-40.
- Hara, M., Yanagihara, T., Takada, T., Itoh, M., Matsuno, M., Yamamoto, T. and Kihara, I. (1998). Urinary excretion of podocytes reflects disease activity in children with glomerulonephritis. Am J Nephrol 18(1): 35-41.
- Nakamura, T., Ushiyama, C., Suzuki, S., Hara, M., Shimada, N., Ebihara, I. and Koide, H. (2000). Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15(9): 1379-1383.
- Niksic, M., Slight, J., Sanford, J. R., Caceres, J. F. and Hastie, N. D. (2004). The Wilms’ tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum Mol Genet 13 (4): 463-471.
- Ohsaki, H., Sofue, T., Kawakami, K., Nishijima, Y., Hara, T., Matsunaga, T., Kushida, Y., Haba, R., Shigematsu, Y., Irino, S. and Norimatsu, Y. (2016). WT1 immunoenzyme staining using SurePathTM processed urine cytology helps to detect kidney disease. Cytopathology 27(1): 43-49.
- Skoog, L. and Tani, E. (2011). Immunocytochemistry: an indispensable technique in routine cytology. Cytopathology 22(4): 215-229.
- Zhang. J., Hansen, K. M., Pippin, J. W., Chang, A. M., Taniguchi, Y., Krofft, R. D., Pickering, S. G., Liu, Z. H., Abrass, C. K. and Shankland, S. J. (2012). De novo expression of podocyte proteins in parietal epithelial cells in experimental aging nephropathy. Am J Physiol Renal Physiol 302(5): 571-580.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ohsaki, H., Matsunaga, T., Fujita, T., Tokuhara, Y., Kamoshida, S. and Sofue, T. (2018). Quantifying Podocytes and Parietal Epithelial Cells in Human Urine Using Liquid-based Cytology and WT1 Immunoenzyme Staining. Bio-protocol 8(9): e2827. DOI: 10.21769/BioProtoc.2827.
Category
Cell Biology > Cell staining > Protein
Cell Biology > Cell-based analysis > Immunocytochemistry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link