- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cobblestone Area-forming Cell Assay of Mouse Bone Marrow Hematopoietic Stem Cells
Published: Vol 8, Iss 9, May 5, 2018 DOI: 10.21769/BioProtoc.2824 Views: 10887
Reviewed by: Giusy TornilloNandini MondalAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Murine Osteoblast and Osteoclast Co-culture on Demineralized Bone Paper for Bone Remodeling
Seema Amin [...] Jungwoo Lee
Jun 5, 2025 2222 Views

Assessing Human Treg Suppression at Single-Cell Resolution Using Mass Cytometry
Jonas Nørskov Søndergaard [...] James B. Wing
Aug 20, 2025 2908 Views

Isolation and Co-culture of Paneth Cells and Intestinal Stem Cells
Ryosuke Isotani [...] Toshimasa Yamauchi
Sep 20, 2025 3460 Views
Abstract
Bone Marrow Hematopoietic Stem Cells (HSCs) require bone marrow microenvironment for their maintenance and proliferation. Culture of Bone Marrow Mesenchymal Stromal Cells (MSCs) provides appropriate environmental signals for HSCs survival in vitro. Here, we provide a detailed protocol that describes culture conditions for MSCs, flow cytometric isolation of HSCs from mouse bone marrow, and perform co-culture of MSCs and HSCs known as Cobblestone area-forming cell (CAFC) assay. Altogether, CAFC assays can be used as a high-throughput in vitro screening model where efforts are made to understand and develop therapies for complex bone marrow diseases. This protocol needs 3 to 4 weeks starting from culturing MSCs, isolating LSK cells (HSCs), and to performing limited dilution CAFC assay.
Keywords: Mesenchymal Stromal CellsBackground
The proliferative, survival and differentiation potential of HSCs is very much dependent on its microenvironment also known as niche. The bone marrow MSCs support the HSCs to keep them in a quiescent state in the bone marrow niche. The intrinsic and extrinsic signals received by the niche contribute to the differentiation of HSCs into mature blood-cell lineages also known as hematopoiesis, without inducing aberrant expansion (Yoshihara et al., 2007; Spindler et al., 2014; Hu et al., 2016). The Cobblestone-Area-Forming Cell Assay (CAFC Assay) is an in vitro co-culture assay of long-term bone marrow HSCs and MSCs. While MSCs are cultured to complete confluence in a tissue culture dish, HSCs are plated over MSCs (de Haan and Ploemacher, 2002). CAFC assays are comparable to in vivo studies of bone marrow and can be used as a rapid screening assay to test the stem cell activity of HSCs and supportive activity of MSCs (Ploemacher et al., 1989). There is a high demand for high throughput screening models that reflect the complex physiology or pathology of the bone marrow microenvironment both in native state or disease models respectively. In this regard, large scale screening was made possible using the HSCs-stroma co-culture system to identify small-molecule inhibitors to develop an effective therapy for acute leukemia (Hartwell et al., 2013). Further, a co-culture system was applied as a model to study several diseases like Fanconi anemia (FA), where it was identified that FA MSCs produce elevated levels of metabolites like glycerophospholipids, which can skew the normal HSCs function (Amarachintha et al., 2015). Further, MSCs provided an impaired environment for HSCs proliferation in patients with aplastic anemia suffering with pancytopenia and in the patients who are in remission after immunosuppression (Schrezenmeier et al., 1996). Further, MSCs from T-cell lymphocytic leukemia mouse model showed adverse proliferation and differentiation capacity of HSCs (Lim et al., 2016). However, in Cord Blood transplants, Cord Blood-MSC co-culture holds promise for successful expansion of cord blood and surge the engraftment in recipients (Denning-Kendall et al., 2003; Robinson et al., 2006). Although several theories were proposed to characterize the isolation and culture of MSCs, ‘The International Society for Cellular Therapy’ has set the minimal criteria for defining ‘multipotent mesenchymal stromal cells’. MSCs derived from bone marrow must be plastic-adherent in standard culture conditions, express cell surface markers, and must differentiate to osteoblasts, adipocytes, and chondroblasts in vitro (Dominici et al., 2006; Keating, 2012). Adhering to these principles, we identified a simplistic approach to culture MSCs from mouse bone marrow and performed limited dilution CAFC assay to enable rapid screenings.
Materials and Reagents
- Pipette tips (USA Scientific, catalog number: 1126-7810 )
- 1,000 µl large orifice pipette tip (USA Scientific, catalog number: 1011-9000 )
- BD Precision glide needles (BD, catalog number: 305155 )
- BD Slip Tip Sterile Syringe 1 ml (BD, catalog number: 309659 )
- BD Single-use Needles 22 G (BD, catalog number: 305159 )
- Falcon 15 ml conical centrifuge tubes (Corning, Falcon®, catalog number: 352097 )
- Falcon standard tissue culture dishes (100 mm culture dish) (Corning, Falcon®, catalog number: 353003 )
- Falcon 35 mm TC-treated Easy-Grip style cell culture dish (Corning, Falcon®, catalog number: 353001 )
- Nunc Edge 2.0 96-well cell culture plates (Thermo Fischer Scientific, Thermo ScientificTM, catalog number: 167425 )
- 3-well chamber slide (IBIDI, catalog number: 80381 )
- Falcon Polystyrene Microplates 6-well plate TC-treated (Corning, Falcon®, catalog number: 353934 )
- C57BL/6J mice of age 4 to 8 weeks old (THE JACKSON LABORATORY, catalog number: 000664 )
- 70% ethanol
- Red blood cell lysis buffer (Sigma-Aldrich, Roche Diagnostics, catalog number: 11814389001 )
- Trypsin-EDTA (0.25%), phenol red (Thermo Fischer Scientific, GibcoTM, catalog number: 25200056 )
- Mouse MSC Functional Identification Kit containing antibodies Osteopontin, Fabp4, and Collagen II (R&D Systems, catalog number: SC010 )
- DPBS (10x), no calcium, no magnesium (Thermo Fischer Scientific, GibcoTM, catalog number: 14200075 )
- 16% formaldehyde (w/v), methanol-free (Thermo Fischer Scientific, Thermo ScientificTM, catalog number: 28906 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9418 )
- 4’,6-Diamidino-2-phenylindole (DAPI) (Thermo Fischer Scientific, InvitrogenTM, catalog number: D1306 )
- VECTASHIELD Antifade mounting medium (Vector Laboratories, catalog number: H-1000 )
- Ficoll-Paque PLUS (GE Healthcare, catalog number: 17144002 )
- Streptavidin APC-Cy-7 (BD, BD Biosciences, catalog number: 554063 )
- PE rat anti-mouse Ly-6A/E (BD, BD Biosciences, catalog number: 561076 )
- Rat anti-mouse CD117 (BD, BD Biosciences, catalog number: 561074 )
- V450 mouse lineage antibody cocktail (BD, BD Biosciences, catalog number: 561301 )
- Fetal bovine serum, qualified, heat inactivated, USDA-approved (FBS) (Thermo Fischer Scientific, GibcoTM, catalog number: 10438034 )
- Iscove’s modified Dulbecco’s medium (Thermo Fischer Scientific, InvitrogenTM, catalog number: 12440053 )
- Bovine calf serum (GE Healthcare, Hyclone, catalog number: SH30072.03 )
- Epidermal growth factor (R&D Systems, catalog number: 2028-EG-200 )
- Platelet-Derived growth factor (R&D Systems, catalog number: 220-BB-010 )
- Penicillin-streptomycin (Thermo Fischer Scientific, GibcoTM, catalog number: 15140122 )
- 2-Mercaptoethanol (Thermo Fischer Scientific, GibcoTM, catalog number: 21985023 )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418 )
- Donkey serum (Sigma-Aldrich, catalog number: D9663 )
- Bone marrow wash buffer (see Recipes)
- MSCs media (see Recipes)
- DPBS blocking solution (see Recipes)
Equipment
- ErgoOne 100-1,000 µl Single Channel Pipettes (USA Scientific, model: 7110-1000 )
- Eppendorf 5804 Benchtop Centrifuge (Eppendorf, model: 5804 )
- BD FACSCanto II to analyze the hematopoietic stem cells (BD, model: BD FACSCanto II )
- BD FACSAria II to sort the hematopoietic stem cells (BD, model: BD FACSAria II )
- NIKON Eclipse 90i microscope to capture immunofluorescence images (Nikon Instruments, model: Eclipse 90i )
- OLYMPUS IX53 Inverted Microscope to capture phase contrast images of cobblestone area (Olympus, model: IX53 )
Software
- BD FACSDiva v8.0.1 Software (BD Biosciences)
- FlowJo software (FLOW JO)
- CellSens software (Olympus)
- GraphPad Prism software
Procedure
- Isolation and culture of Mouse Bone Marrow Mesenchymal Stromal Cells
- Euthanize an adult C57BL/6 mouse by CO2 asphyxiation in accordance with Institutional Animal Care and Use Committee (IACUC) protocol. Spray the mouse with 70% ethanol to disinfect. Incise the skin at hind leg portion and expose the muscles. Cut the pelvic joint and remove the hind leg. Cut the knee joint to separate femur from the lower portion of the hind limb. Remove the muscles of the femur and cut the ends of the femur to enable flushing.
- Transfer the femur into ice-cold wash buffer and flush gently with ice-cold wash buffer using a 1 ml syringe with a 22 G needle until all the cells are removed and the bone appears white. Repeat the same procedure for the other hind limb and combine the cells from both femurs.
- Spin BM cells in a 15 ml conical tube at 500 x g for 5 min at room temperature.
- Lyse RBCs using red blood cell lysis buffer.
- To lyse RBCs, suspend the cells obtained from two femurs in 2 ml wash buffer and add 4 ml of red blood cell lysis buffer. Mix the cells by inverting few times every min for 10 min.
- Centrifuge the tube at 400 x g for 6 min at room temperature.
- Discard the supernatant and resuspend the BM cells in 10 ml wash buffer and centrifuge at 400 x g for 10 min to remove the lysed red blood cells. Repeat this process for one more time.
- Remove the excess wash buffer from the cell pellet and add 10 ml of MSCs media to the cell pellet. Pipet the media gently to evenly mix the cell pellet.
- Plate 1 x 107 cells in a 100 mm tissue culture dish with 10 ml of MSCs media.
- Incubate the cells at 37 °C with 5% CO2 in a standard humidified tissue culture room incubator.
- Mesenchymal stromal cells start to adhere to the plastic dish while the rest of the BM cells float in the media.
- Replace the media with fresh MSCs media after three days in culture and culture the cells for 10 to 12days.
- During the first media change, most of the suspending cells are removed and MSCs start to adhere to the bottom of the dish.
- Subculture the MSCs from one 100 mm dish into a 35 mm dish using trypsin-EDTA. The cells are devoid of any other types of BM cells by the second passage and are ready for the assay from passage 3 (Figure 1).
- Since MSCs do not have high dividing potential, split the cells into 1 to 2 ratios each time when sub-cultured to subsequent passages and maintain in 35 mm dishes until assay is performed. MSCs can be passaged up to five passages without losing MSC function.
- Cells can be subsequently passaged to 96-well flat-bottom plates when ready to perform the assay.

Figure 1. Mouse Bone Marrow Mesenchymal stromal cells. Plastic adhered MSCs were shown in phase contrast image. Scale bar represents 50 µm.
- Euthanize an adult C57BL/6 mouse by CO2 asphyxiation in accordance with Institutional Animal Care and Use Committee (IACUC) protocol. Spray the mouse with 70% ethanol to disinfect. Incise the skin at hind leg portion and expose the muscles. Cut the pelvic joint and remove the hind leg. Cut the knee joint to separate femur from the lower portion of the hind limb. Remove the muscles of the femur and cut the ends of the femur to enable flushing.
- Characterization of Mouse Bone Marrow Mesenchymal stromal cells
- To obtain pure MSCs, sub-culture plastic adherent cells for at least three passages. MSCs are multipotent cells and can be induced to differentiate into osteoblasts, adipocytes, and chondrocytes. However, MSCs in culture consist of a mix of osteoblasts, adipocytes, and chondrocytes and express markers unique to them. It is critical to evaluate the functionality of MSCs before performing CAFC since the loss of a gene or exposure to various environments can give rise to differences in differentiation of MSCs.
- To identify the multipotential of MSCs, plate cells on chamber slides and stain with antibodies Osteopontin, Fatty acid binding protein 4 (Fabp4), and Collagen II to identify osteoblasts, adipocytes, chondroblasts respectively using the Mouse MSC Functional Identification Kit.
- Immunofluorescence of MSCs: Rinse MSCs once with 1x DPBS and fix with 4% paraformaldehyde (PFA) diluted in 1x DPBS for 10 min at room temperature.
- Permeabilize the cells with 0.2% Triton X-100 made in 1x DPBS for 3 min and block with blocking buffer DPBS blocking solution for 30 min.
- Gently remove the blocking solution and follow overnight incubation at 4 °C with primary antibody. Adipocyte marker: Goat Anti-Mouse FABP4 Antigen-affinity Purified Polyclonal Antibody. Chondrocyte marker: Sheep Anti-Mouse Collagen II Antigen-affinity Purified Polyclonal Antibody. Osteocyte marker: Goat Anti-Mouse Osteopontin Antigen-affinity Purified Polyclonal Antibody. Dilute the primary antibodies to 1:500 in 1x DPBS containing 0.1% BSA.
- Wash the slides with 1x DPBS containing 0.1% BSA for three times at room temperature with each wash for 10 min. Incubate with secondary antibodies diluted to 1:1,000 in 1x DPBS containing 0.1% BSA for at least 2 h at room temperature. Use Donkey Anti-Goat and Donkey Anti-Sheep IgG secondary antibodies.
- Wash the slides with 1x DPBS containing 0.1% BSA for three times at room temperature with each wash for 10 min.
- After the final wash, add the nuclear dye DAPI at 1:2,000 in 1x DPBS for 5 min to evaluate nuclear morphology.
- Wash further 3 times with 1x DPBS for 5 min each wash.
- Remove excess DPBS and mount slides with Vectashield mounting media.
- Acquire images using any immunofluorescence microscope at 20x objective (Figure 2).
- The ratio of adipocytes:chondrocytes:osteocytes from a wildtype C57BL/6J mice is 60:20:20.
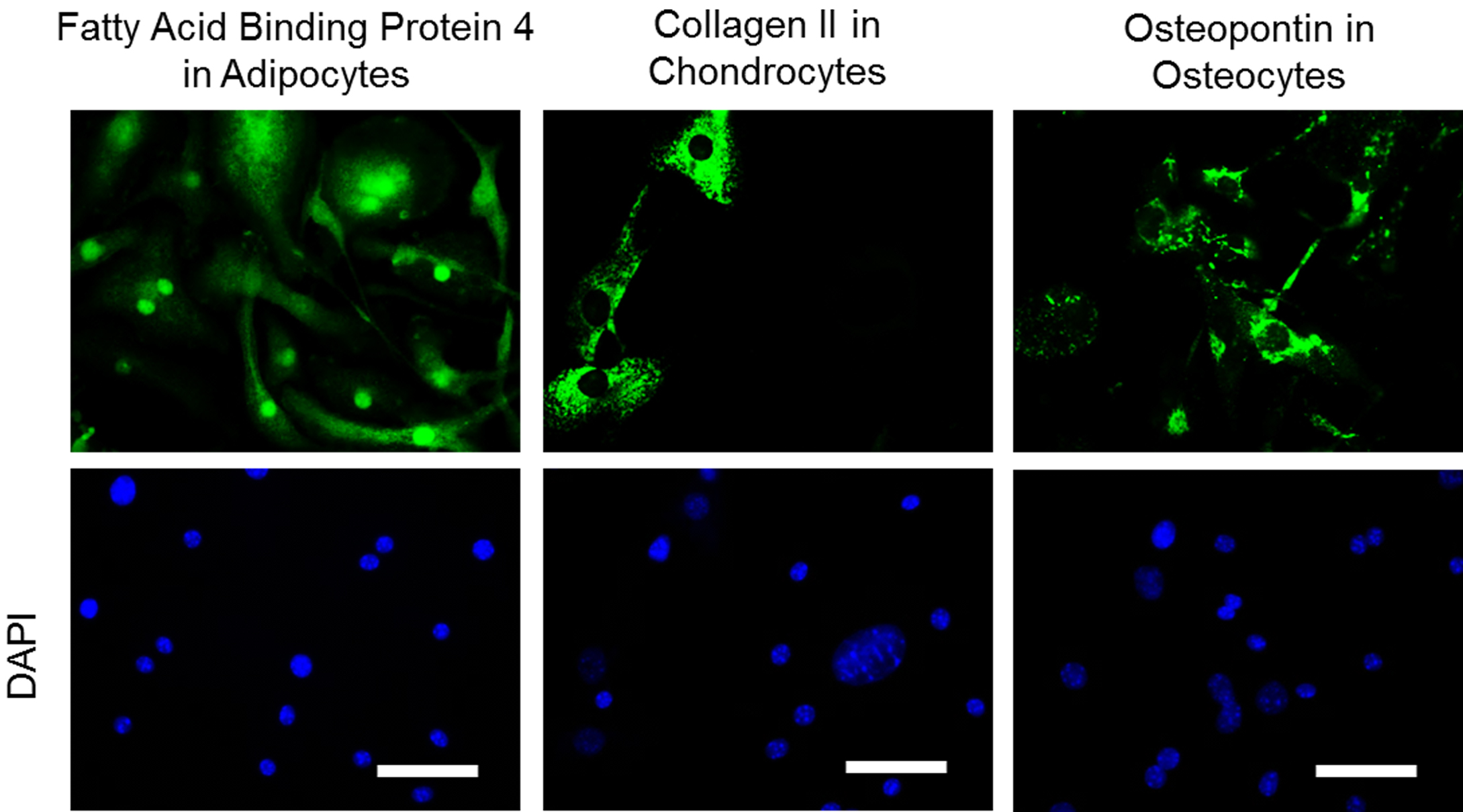
Figure 2. Representative images of Mouse BM MSCs stained with antibodies Fabp4, Collagen II, and Osteopontin to identify adipocytes, chondroblasts, and osteoblasts lineage cells respectively present in bone marrow derived mesenchymal stromal cells. Scale bar represents 10 µm.
- To obtain pure MSCs, sub-culture plastic adherent cells for at least three passages. MSCs are multipotent cells and can be induced to differentiate into osteoblasts, adipocytes, and chondrocytes. However, MSCs in culture consist of a mix of osteoblasts, adipocytes, and chondrocytes and express markers unique to them. It is critical to evaluate the functionality of MSCs before performing CAFC since the loss of a gene or exposure to various environments can give rise to differences in differentiation of MSCs.
- Isolation of Mouse Bone Marrow Hematopoietic stem cells (HSCs)
- Isolate HSCs from Bone marrow mononuclear cells (BMMCs) using flow cytometry after staining with cell surface markers negative for Lineage cocktail and positive for Sca-1, c-Kit (Lineage Negative, Sca-1 Positive, c-Kit Positive, LSK). HSCs are also termed as LSKs.
- Obtain BM cells from mouse femurs by gently flushing cells out the bones using wash buffer.
- Wash BM cells twice with wash buffer by centrifugation at 500 x g for 5 min at room temp.
- Resuspend the cells with 3 ml of wash buffer and gently overlay on 6 ml of Ficoll-Paque PLUS in a 15 ml conical tube.
- Carefully layer the diluted cell sample onto the Ficoll-Paque PLUS solution and take precautions not to mix the cells and Ficoll.
- Centrifuge at 400 x g for 30 min at room temperature with slow acceleration and brake turned off.
- Pipette out upper layer containing plasma and platelets using a sterile pipette, leaving the BMMCs layer undisturbed at the interface.
- Transfer the layer of BMMCs to a sterile centrifuge tube using a sterile pipette.
- Wash the BMMCs with wash buffer once.
- Pellet BMMCs and first stain with a lineage (Lin) cocktail of antibodies (biotinylated anti-mouse antibodies directed against CD3e, CD11b, CD45R/B220, Gr-1, and Ter119). Add 2 μl of each antibody to stain one million BMMCs. Incubate the cells with antibodies at 4 °C for 20 min.
- Wash the cells with bone marrow wash buffer one time and pellet the cells.
- Further, add 5 μl of fluorochrome-conjugated Streptavidin to bind to lineage antibodies.
- Wash BMMCs once with buffer and pellet the cells. Further, add 2 μl of Sca-1 and c-kit conjugated with fluorochrome to stain one million BMMCs.
- Wash Triple (Lineage, Sca-1, c-kit) stained BMMCs couple times and suspend one million cells in 1 ml of wash buffer for flow sorting.
- Flow sort on a BD FACS Aria II with a FACSDiva software.
- Analyze the Flow cytometric data to check the quality of LSK cells using the FlowJo software as displayed in pseudo color plot (Figure 3).
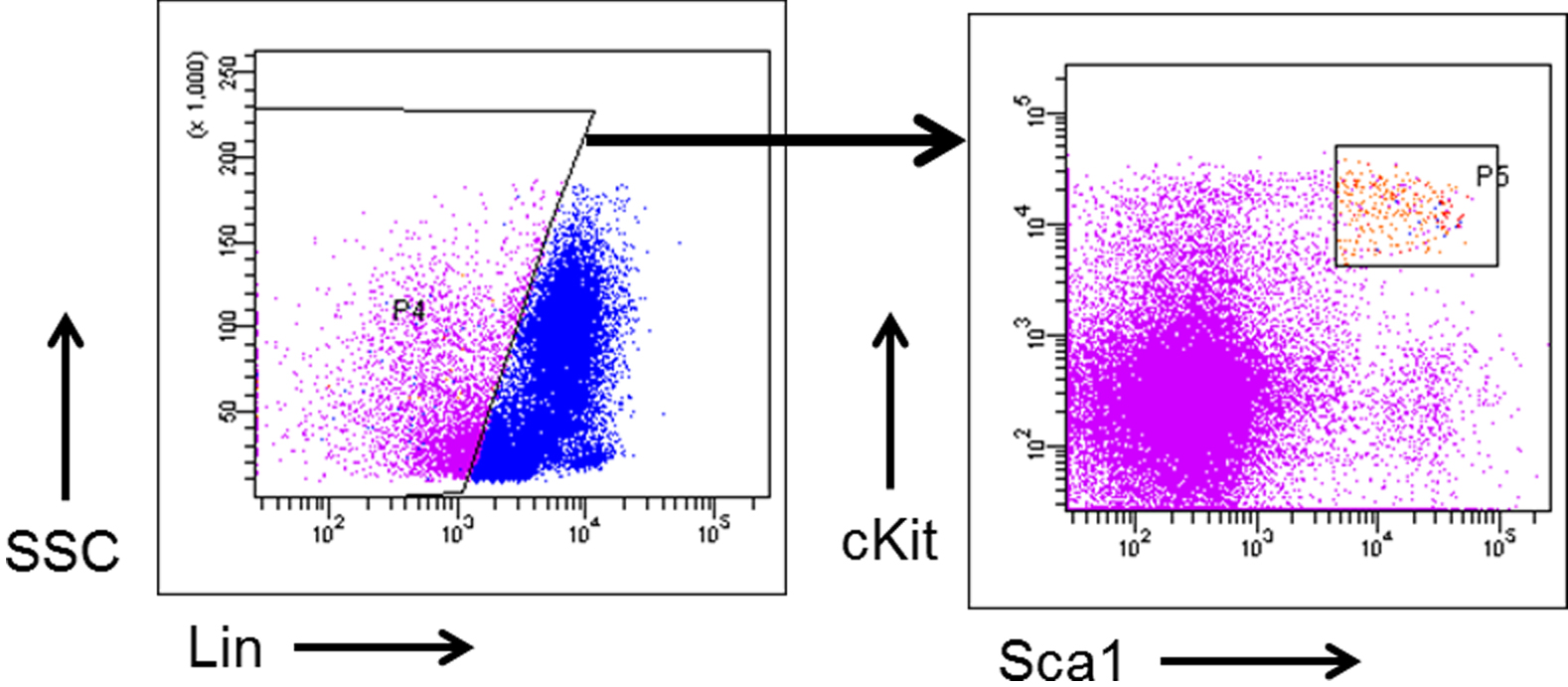
Figure 3. Gating strategy to identify and isolate Hematopoietic stem cells from Mouse Bone Marrow. Bone Marrow cells flushed from mouse femurs were subjected to Ficoll-Paque PLUS separation to isolate Bone Marrow Mono Nuclear Cells and then stained with HSC cell surface markers to identify and cell-sort using the flow cytometer.
- Isolate HSCs from Bone marrow mononuclear cells (BMMCs) using flow cytometry after staining with cell surface markers negative for Lineage cocktail and positive for Sca-1, c-Kit (Lineage Negative, Sca-1 Positive, c-Kit Positive, LSK). HSCs are also termed as LSKs.
- Cobble stone area forming cell assay
- Prior to isolation of LSK cells using flow cytometry, culture MSCs to complete confluence in a 35 mm culture dish or a 6-well plate.
- MSCs between passages 3 to 6 should be used for plating LSK cells.
- On the day of assay, sort LSK cells from Bone marrow as described (Procedure C) using flow cytometry.
- Pellet the isolated LSK cells and resuspend with MSCs media. Replace the media in the culture dish growing MSCs in media suspended with LSK cells. Approximately, plate 2 x 103 LSK cells on confluent MSCs in a 35 mm dish.
- Co-culture the cells at 37 °C, 5% CO2 in a humidified incubator to allow the precursor cells to form hematopoietic clones under the stromal layers.
- Cobblestone areas start to appear as early as 5 days and can be predominantly seen by 7 days.
- Feed the culture two times a week by replacing half of the medium. Image the phase-dark hematopoietic clones using a phase contrast microscope (Figure 4) at 10x objective and installed with software like CellSens software from Olympus.
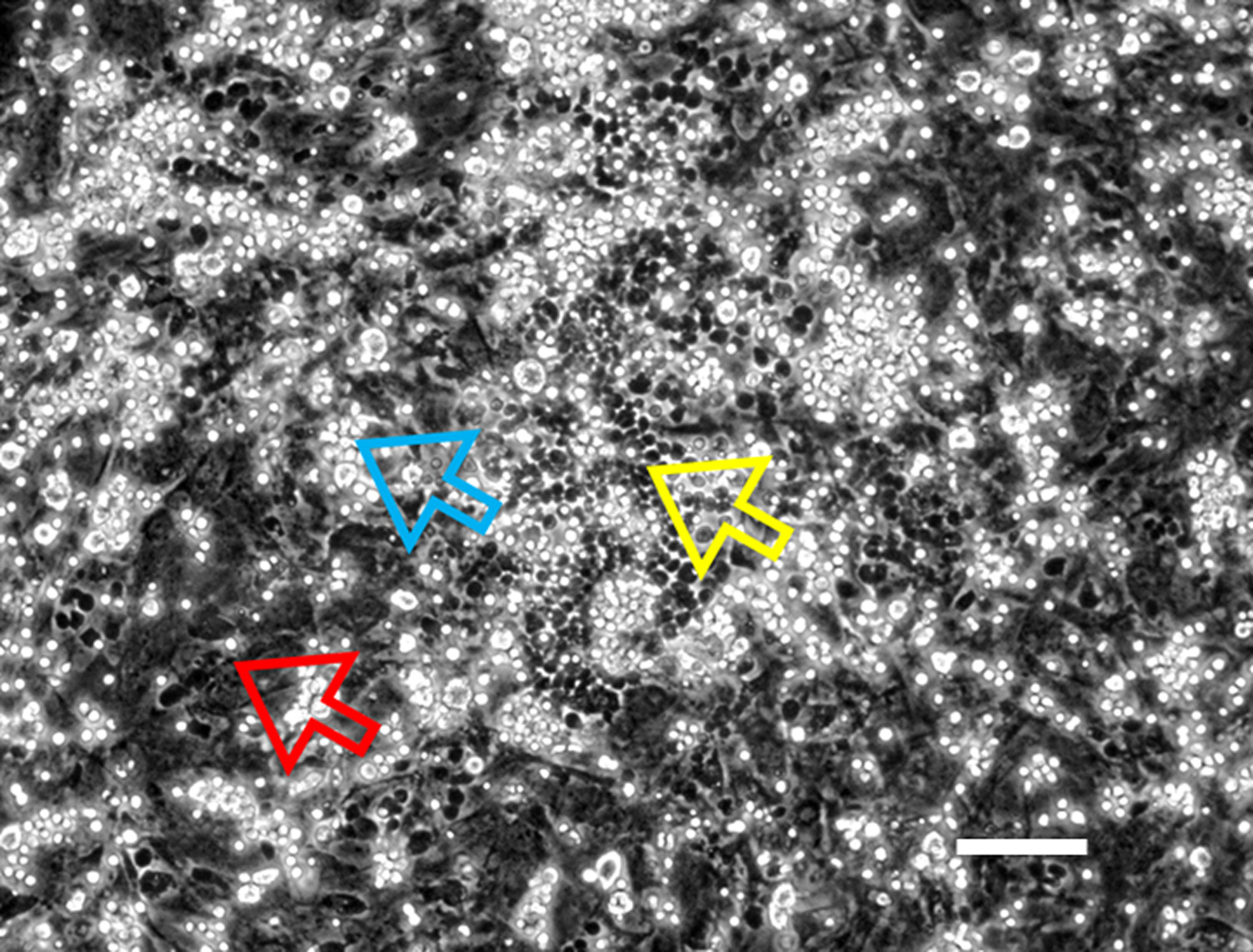
Figure 4. Representative image of Cobblestone area forming cell assay. HSCs are cultured on a confluent layer of MSCs to form the cobblestone areas. Yellow arrow–phase dull cells (true stem cells growing under the MSCs layer), Blue arrow–phase bright cells (stem progenitor cells suspended or loosely attached to MSCs), and Red arrow–MSCs (Confluent cell layer adhered to the plastic dish). Scale bar represents 50 µm.
- Prior to isolation of LSK cells using flow cytometry, culture MSCs to complete confluence in a 35 mm culture dish or a 6-well plate.
- CAFC limited dilution assay
- Limiting dilution assay (LDA) is performed in 96-well flat-bottom plates with confluent MSCs and dilutions of LSK cells (0, 10, 30, 90, 270, and 810) differing with a factor of 3 in each well. Plate MSCs in 10 wells in a flat-bottom 96 well plate per each dilution (Figure 5A). No LSK cells are added to 0 dilution wells and they serve as negative control.
- Perform three different LDA experiments with independently derived MSCs from bone marrows of mice.
- Score the well as ‘positive’ if it contains one or more cobblestone areas and ‘negative’ if contains no cobblestone areas.
- Cobblestone area is at least 6 cells (in proximity to each other) growing underneath the stroma. Although cobblestone-like cells appear as phase dark, these cells appear as nonrefractile in 96-well plates because of the deflection of light.
- Only dilutions with both negative and positive wells are informative for frequency analysis (Figure 5B).
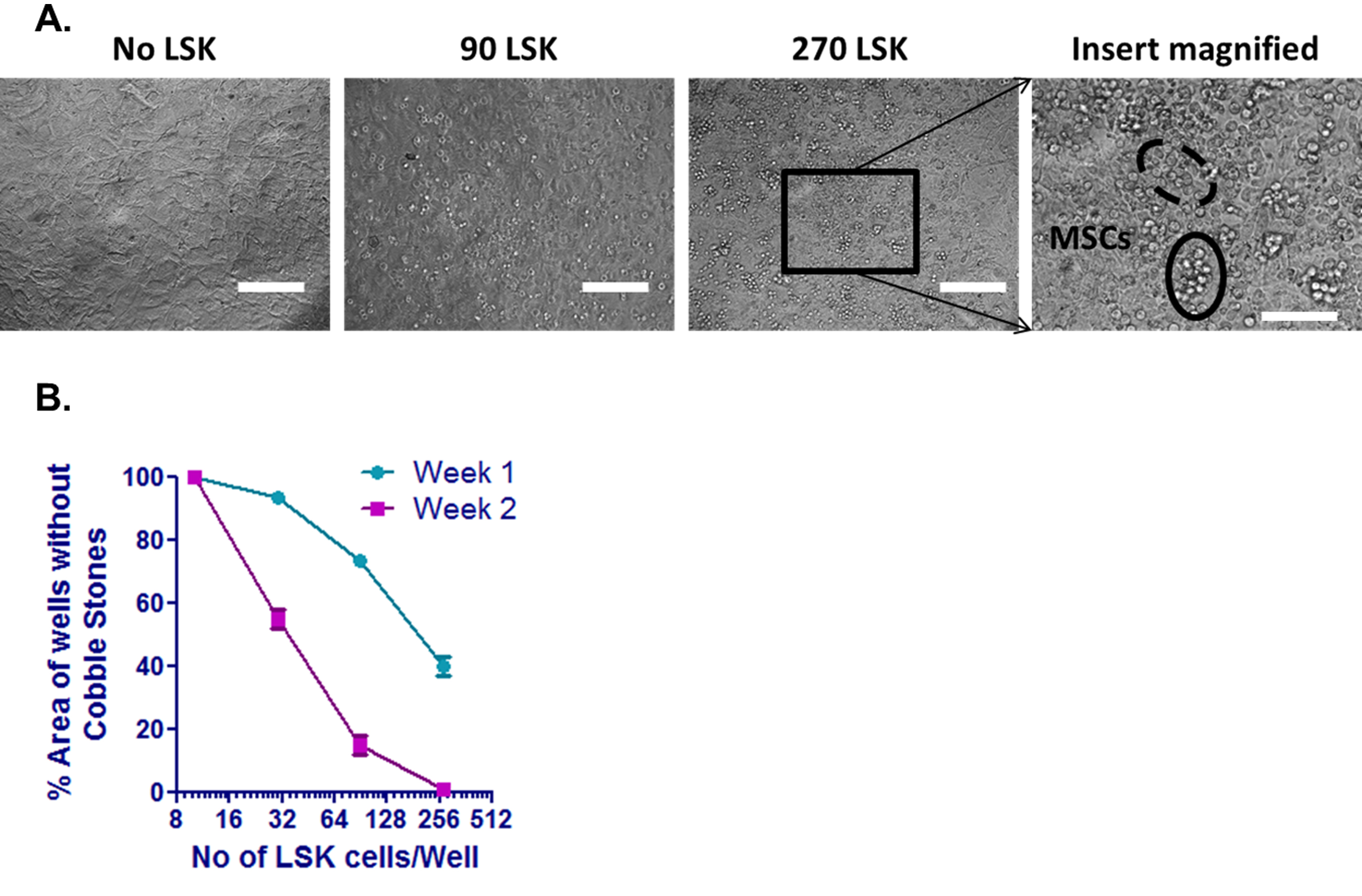
Figure 5. CAFC Limited dilution assay. A. CAFC assay performed in a 96-well flat-bottom plate. Flow sorted LSK cells were counted and plated on confluent MSCs in a flat-bottom 96-well plate. Each assay was performed in triplicates. Representative images of the cobblestones formed in wells with no LSK cells, 90 and 270 LSK cells. Scale bars represent 100 µm. A portion of the cobblestone area was magnified; Scale bar represents 50 µm. Phase dull cells were identified in a dotted circle while phase bright cells were identified in a solid circle. Both phase dull and phase bright cells need to be present to call it a true cobblestone area. B. Phase dull cell areas were measured in each well as shown in above panel. Percentage of the well covered with cobblestone areas was counted and plotted against the no of LSK cells plated in each well. Cultures were maintained for two weeks and the areas were counted at the end of each week.
- Limiting dilution assay (LDA) is performed in 96-well flat-bottom plates with confluent MSCs and dilutions of LSK cells (0, 10, 30, 90, 270, and 810) differing with a factor of 3 in each well. Plate MSCs in 10 wells in a flat-bottom 96 well plate per each dilution (Figure 5A). No LSK cells are added to 0 dilution wells and they serve as negative control.
Data analysis
Plot the charts and analyze data using GraphPad Prism software. Compare the means of two groups in LDA using t-test.
Notes
- MSCs do not have high recovery rate when cryopreserved. So always culture and perform the assay with fresh MSCs.
- To prevent rupture of MSCs and LSK cells while suspending into media, use large orifice pipette tips.
- Perform CAFC assay only when MSCs are grown to complete confluence.
- Add LSK cell surface markers to BMMCs suspended into 200 µl or less volume of buffer.
Recipes
- Bone marrow wash buffer
Dulbecco’s phosphate-buffered saline made to 1x concentration (1x DPBS)
10% FBS - MSCs media
Iscove’s modified Dulbecco’s medium 500 ml Bovine calf serum 20% Epidermal growth factor (rmEGF) 10 ng/ml Platelet-Derived growth factor (rhPDGF) 200 ng/μl Penicillin-streptomycin 1% 2-Mercaptoethanol 10-4 mol/L - DPBS blocking solution
1x DPBS
1% Triton X-100
1% DMSO
1% BSA
1% Serum (Serum from the animal in which the secondary antibodies were raised)
Acknowledgments
This protocol was adapted from previous work published in Hematopoietic Stem Cell Protocols in 2002 by de Haan and Ploemacher. Cell sorting for LSK cells was performed at Research Flow Cytometry Core at Cincinnati Children’s Hospital Medical Center. This investigation was supported by NIH grants R01 HL076712, R01 CA157537 and T32 HL091805. Qishen Pang is supported by a Leukemia and Lymphoma Scholar award.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: S. A. designed and performed research, analyzed data, and wrote the paper; Q. P. designed research, contributed vital new reagents, analyzed data.
References
- Amarachintha, S., Sertorio, M., Wilson, A., Li, X. and Pang, Q. (2015). Fanconi anemia mesenchymal stromal cells-derived glycerophospholipids skew hematopoietic stem cell differentiation through Toll-like receptor signaling. Stem Cells 33(11): 3382-3396.
- de Haan, G. and Ploemacher, R. (2002). The cobblestone-area-forming cell assay. In: Hematopoietic Stem Cell Protocols. In: Klug, C. A. and Jordan, C. T. (Eds). Humana Press, Totowa 143-151.
- Denning-Kendall, P., Singha, S., Bradley, B. and Hows, J. (2003). Cobblestone area-forming cells in human cord blood are heterogeneous and differ from long-term culture-initiating cells. Stem Cells 21(6): 694-701.
- Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D. and Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4): 315-317.
- Hartwell, K. A., Miller, P. G., Mukherjee, S., Kahn, A. R., Stewart, A. L., Logan, D. J., Negri, J. M., Duvet, M., Jaras, M., Puram, R., Dancik, V., Al-Shahrour, F., Kindler, T., Tothova, Z., Chattopadhyay, S., Hasaka, T., Narayan, R., Dai, M., Huang, C., Shterental, S., Chu, L. P., Haydu, J. E., Shieh, J. H., Steensma, D. P., Munoz, B., Bittker, J. A., Shamji, A. F., Clemons, P. A., Tolliday, N. J., Carpenter, A. E., Gilliland, D. G., Stern, A. M., Moore, M. A. S., Scadden, D. T., Schreiber, S. L., Ebert, B. L. and Golub, T. R. (2013). Niche-based screening identifies small-molecule inhibitors of leukemia stem cells. Nat Chem Biol 9(12): 840-848.
- Hu, X., Garcia, M., Weng, L., Jung, X., Murakami, J. L., Kumar, B., Warden, C. D., Todorov, I. and Chen, C. C. (2016). Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat Commun 7: 13095.
- Keating, A. (2012). Mesenchymal stromal cells: new directions. Cell Stem Cell 10(6): 709-716.
- Lim, M., Pang, Y., Ma, S., Hao, S., Shi, H., Zheng, Y., Hua, C., Gu, X., Yang, F., Yuan, W. and Cheng, T. (2016). Altered mesenchymal niche cells impede generation of normal hematopoietic progenitor cells in leukemic bone marrow. Leukemia 30(1): 154-162.
- Ploemacher, R. E., van der Sluijs, J. P., Voerman, J. S. and Brons, N. H. (1989). An in vitro limiting-dilution assay of long-term repopulating hematopoietic stem cells in the mouse. Blood 74(8): 2755-2763.
- Robinson, S. N., Ng, J., Niu, T., Yang, H., McMannis, J. D., Karandish, S., Kaur, I., Fu, P., Del Angel, M., Messinger, R., Flagge, F., de Lima, M., Decker, W., Xing, D., Champlin, R. and Shpall, E. J. (2006). Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant 37(4): 359-366.
- Schrezenmeier, H., Jenal, M., Herrmann, F., Heimpel, H. and Raghavachar, A. (1996). Quantitative analysis of cobblestone area-forming cells in bone marrow of patients with aplastic anemia by limiting dilution assay. Blood 88(12): 4474-4480.
- Spindler, T. J., Tseng, A. W., Zhou, X. and Adams, G. B. (2014). Adipocytic cells augment the support of primitive hematopoietic cells in vitro but have no effect in the bone marrow niche under homeostatic conditions. Stem Cells Dev 23(4): 434-441.
- Yoshihara, H., Arai, F., Hosokawa, K., Hagiwara, T., Takubo, K., Nakamura, Y., Gomei, Y., Iwasaki, H., Matsuoka, S., Miyamoto, K., Miyazaki, H., Takahashi, T. and Suda, T. (2007). Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1(6): 685-697.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Amarachintha, S. and Pang, Q. (2018). Cobblestone Area-forming Cell Assay of Mouse Bone Marrow Hematopoietic Stem Cells. Bio-protocol 8(9): e2824. DOI: 10.21769/BioProtoc.2824.
Category
Stem Cell > Adult stem cell > Hematopoietic stem cell
Cell Biology > Cell isolation and culture > Co-culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








