- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of Mesenchymal Stem Cells Attachment to Endothelial Cells
Published: Vol 8, Iss 6, Mar 20, 2018 DOI: 10.21769/BioProtoc.2776 Views: 8221
Reviewed by: Chao WangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Full Good Manufacturing Practice–Compliant Protocol for Corneal Stromal Stem Cell Cultivation
Mithun Santra [...] Gary H.F. Yam
Sep 20, 2024 2151 Views

Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs
Quang Bach Le [...] Deepak Choudhury
Nov 20, 2025 1536 Views

A Protocol to Induce Brown and Beige Adipocyte Differentiation From Murine and Human Adipose-Derived SVF
Rohit Raj Yadav [...] Narendra Verma
Dec 5, 2025 1642 Views
Abstract
Mesenchymal stem cells (MSCs) have shown profound therapeutic potential in tissue repair and regeneration. However, recent studies indicate that MSCs are largely entrapped in lungs after intravenous delivery and die shortly. The underlying mechanisms have been poorly understood. We have provided evidence to show that excess expression and activation of integrins in culture-expanded MSCs is a critical cause of MSCs adhesion to endothelial cells of the lung microarteries resulting in the entrapment of the cells (Wang et al., 2015). Therefore, it may be meaningful to test the adhesive ability of MSCs to endothelial cells in vitro before intravenous administration to avoid their lung vascular obstructions. Here we report a simple method to measure MSCs attachment to endothelial cells.
Keywords: Mesenchymal stem cellsBackground
Mesenchymal stem cells (MSCs) are emerging as an extremely promising therapeutic agent, and numerous clinical trials for a variety of diseases are underway (Salem and Thiemermann, 2010). Intravenous infusion of MSCs has been a popular delivery route for MSCs therapies in recent clinical trials because of its convenience and safety (Wu and Zhao, 2012). However, increasing evidence has indicated that MSCs cause considerable vascular obstructions following intravascular injection. Upon intravenous infusion, more than 80% of MSCs are entrapped in the lungs, and only less than 1% of MSCs are detected in the acute ischemic heart or brain (Lee et al., 2009; Toma et al., 2009).
Recent studies suggest that MSCs are largely stuck in the precapillary microvessel after intravenous administration and most of them die shortly of local ischemia (Toma et al., 2009). Therefore, it has become an increasing concern over the safety and efficacy of intravascularly administered MSCs. The mechanisms of vascular obstructions of MSCs have not been fully understood.
Our data have indicated that excess expression of integrins in MSCs is an important cause for their lung entrapment, which leads to attachment of the cells to endothelial cells in the lungs, thus reducing their trafficking and homing to inflamed tissues. Functional blockade of integrins in MSCs, especially after integrin β1 blockade, significantly decreases their attachment to endothelial cells, resulting in a substantial reduction of MSCs entrapped in the lungs, elevated levels of circulating MSCs in the blood, and increased engraftment of the cells to inflamed tissues (Wang et al., 2015). Here we provide a methodology for measuring the attachment of MSCs to endothelial cells in vitro.
Materials and Reagents
- 24-well plates (Corning, Costar®, catalog number: 3524 )
- 12-well plates (Corning, catalog number: 3512 )
- 10 cm plates (Corning, catalog number: 353003 )
- Pipette (Corning, catalog number: 4100 )
- 15- or 50-ml conical centrifuge tubes (Corning, catalog number: 430052 , 430828 )
- Human bone marrow-derived MSCs (Lonza, catalog number: PT-2501 )
- Human umbilical vein endothelial cells (HUVECs) (Lonza, catalog number: CC-2517 )
- Human lung microvascular endothelial cells (HMVECs-L) (Lonza, catalog number: CC-2527 )
- Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 41966052 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, Gibco TM, catalog number: 10270106 )
- Penicillin-streptomycin (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- EGM-2 MV SingleQuot Kit Supplements & Growth Factors (Lonza, catalog number: CC-4147 )
- EGMTM-2 MV BulletKitTM Medium (Lonza, catalog number: CC-3202 )
- Endothelial basal medium-2 (EBM-2) (Lonza, catalog number: CC-3156 )
- Fibronectin (Sigma-Aldrich, catalog number: F0556 )
- Sterile phosphate buffer saline (PBS), pH 7.2 (Thermo Fisher Scientific, GibcoTM, catalog number: 20012068 )
- Hank’s balanced salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14025092 )
- Vitronectin (Sigma-Aldrich, catalog number: V8379 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A2153 )
- Lipophilic fluorophore 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI) (Sigma-Aldrich, catalog number: 468495 )
- Trypsin-EDTA (0.25%), phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- Mouse anti-human integrin β1 antibody (Merck, catalog number: MAB1987 )
- Mouse anti-human integrin β1 activated antibody (Merck, catalog number: MAB2079Z )
- Anti-human integrin α5 (Merck, catalog number: MAB1956Z )
- Anti-human CD51/CD61 (integrin αVβ3) purified (Thermo Fisher Scientific, eBioscienceTM, catalog number: 14-0519 )
- Mouse isotype IgG (Sigma-Aldrich, catalog number: M6898 )
- Tumor necrosis factor-α (TNF-α) (PeproTech, catalog number: 300-01A )
- Medium 199 (Sigma-Aldrich, catalog number: M4530 )
- Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: P6148 )
- Ficoll-paque Plus solution (GE Healthcare, catalog number: 17-1440-02 )
- Dead Cell Apoptosis Kit with Annexin V Alexa FluorTM 488 & Propidium Iodide (PI) (Thermo Fisher Scientific, InvitrogenTM, catalog number: V13241 )
- Human leucocytes (see Recipes)
Equipment
- Traceable Nano Timer (Fisher Scientific, catalog number: 14-649-83 )
- Centrifuge (Eppendorf, catalog number: 5810 R )
- Hemocytometer (Hirschmann Instruments, catalog number: 8100103 )
- CO2 incubator (Panasonic, model: MCO-19AIC (UV))
- Fluorescence microscope (Leica Microsystems, catalog number: Leica DMI6000 B )
Procedure
- Cell culture and single cell suspensions
- hMSCs culture
Human bone marrow-derived MSCs (hMSCs) were purchased from Lonza. Culture hMSCs in a growth medium consisting of DMEM, 10% FBS, and 1% penicillin and streptomycin for 72 h.
- Culture of human umbilical vein endothelial cells (HUVECs) and human lung microvascular endothelial cells (HMVECs-L)
- Culture HUVECs in endothelial growth medium (EGM)-2 plus 2% FBS and supplements for 72 h.
- Culture HMVECs-L in EGMTM-2 MV BulletKitTM Medium for 72 h.
Note: EGMTM-2 MV BulletKitTM Medium includes endothelial basal medium-2 (EBM-2, Lonza) and the following growth supplements: human epidermal growth factor (hEGF); vascular endothelial growth factor (VEGF); R3-insulin-like growth factor-1 (R3-IGF-1); ascorbic acid; hydrocortisone; human fibroblast growth factor-Beta (hFGF-β); fetal bovine serum (FBS) and gentamicin/amphotericin-B (GA).
- Culture HUVECs in endothelial growth medium (EGM)-2 plus 2% FBS and supplements for 72 h.
- hMSCs culture
- Optimization of blocking antibody concentrations and cell adhesion assay
- Coat 24-well plates with 10 μg/ml fibronectin (diluting in sterile HBSS or PBS solutions) or 0.4-1 μg/ml vitronectin (diluting in sterile water) for 1 h at room temperature.
- Remove fibronectin or vitronectin buffer without washing, air dry for 1-2 h in a hood.
- Block the plates with 3% BSA in PBS for 1 h at 37 °C.
- Gently wash the plates with PBS once, air dry in a hood.
- Use 3% BSA in PBS alone-coated wells as a negative control.
- Pre-label hMSCs and freshly isolated human peripheral blood leucocytes with fluorescent label DiI (Lipophilic fluorophore 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate) (Wu et al., 2006).
- For cells in suspension, wash with PBS and incubate with DiI at a concentration 2 μg/ml in DMEM basal medium (serum-free) for 20-30 min at 37 °C. Then incubate with fresh growth medium (DMEM supplemented with 10% FBS) for 30 min. After two washes with PBS (400 x g, 5 min), re-suspend the cells in fresh growth medium.
- For cells in adhesion, wash with PBS and incubate with DiI at a concentration of 2 μg/ml in DMEM basal medium (serum-free) for 20-30 min at 37 °C. Then replace the medium with fresh growth medium and incubate for 30 min. Trypsinize the cells with 0.25% trypsin-EDTA for 2 min and suspend cells in DMEM supplemented with 10% FBS.
- For cells in suspension, wash with PBS and incubate with DiI at a concentration 2 μg/ml in DMEM basal medium (serum-free) for 20-30 min at 37 °C. Then incubate with fresh growth medium (DMEM supplemented with 10% FBS) for 30 min. After two washes with PBS (400 x g, 5 min), re-suspend the cells in fresh growth medium.
- Pre-incubate hMSCs in suspension with different concentrations of integrin β1, integrin α5 or integrin αVβ3 blocking to determine the lowest concentrations of the blocking antibodies that achieve maximum inhibition to hMSCs attachment (Wang et al., 2015).
- Incubate hMSCs with anti-integrin β1 blocking mAb at concentrations of 0, 2, 4, 6, 8, 10 and 20 μg/ml in DMEM containing 2% FBS for 30 min at 37 °C.
- Incubate hMSCs with anti-integrin α5 blocking mAb at concentrations of 0, 2.5, 5, 10, 20 and 40 μg/ml in DMEM containing 2% FBS for 30 min at 37 °C.
- Incubate hMSCs with anti-integrins αVβ3 blocking mAb at concentrations of 0, 2.5, 5, 10 and 20 μg/ml for 30 min at 37 °C.
- Incubate hMSCs with isotype IgG antibody as a control.
- Wash once with PBS after incubations.
- Incubate hMSCs with anti-integrin β1 blocking mAb at concentrations of 0, 2, 4, 6, 8, 10 and 20 μg/ml in DMEM containing 2% FBS for 30 min at 37 °C.
- Seed 5 x 104 per well of hMSCs after blockade with integrin β1, integrin α5 or isotype control IgG, or 5 x 104 per well freshly isolated human peripheral blood leucocytes on fibronectin-coated plates.
- Seed 5 x 104 hMSCs per well after blockade with integrin αVβ3 or isotype control IgG on vitronectin-coated plates.
- Incubate the cells in DMEM plus 10% FBS for 30, 60, 90 and 120 min at 37 °C.
- Collect non-adherent cells every 30 min (wash once with PBS).
- Centrifuge non-adherent cells at 400 x g, 5 min.
- Count the non-adherent cells with a hemocytometer.
- Photograph the DiI-labelled hMSCs in attachment by fluorescence microscopy.
- The experiment must be performed in quadruplicate for each variable.
- Coat 24-well plates with 10 μg/ml fibronectin (diluting in sterile HBSS or PBS solutions) or 0.4-1 μg/ml vitronectin (diluting in sterile water) for 1 h at room temperature.
- hMSCs attachment to endothelial cells
- Culture HUVECs and HMVECs-L in 12-well plates with endothelial growth medium (EGM)-2 and EGMTM-2 MV BulletKitTM Medium, respectively, to confluence. The confluence density is approximate percentage of 80%-90%. Cells were treated with or without of 10 ng/ml TNF-α for 24 h immediately before the addition of hMSCs or leucocytes. TNF-α treatment, which is able to activate endothelial cells (Ko et al., 2009), increased the attachment of hMSCs and leucocytes to endothelial cells.
- Isolate leucocytes from freshly human peripheral blood (Boyum, 1976; Chacko et al., 2013).
- Prepare hMSCs: trypsinize hMSCs from 80%-90% confluent 10 cm plates.
- Label hMSCs and leucocytes with fluorescent DiI.
- Suspend DiI-labeled leucocytes at a density of 1 x 106 in M199 plus 0.1% BSA.
- Suspend DiI-labeled hMSCs at a density of 0.5 x 106 in DMEM plus 0.1% BSA.
- Find out the appropriate time-point to detect hMSCs attachment to endothelial cells.
- Add DiI-labeled hMSCs to 80%-90% confluent monolayers of HUVECs and HMVECs-L in 12-well plates and incubate cells at 37 °C and 5% CO2.
- Detect hMSCs attached to endothelial cells every 15 min for 60 min.
- Incubation for 30 min is normally sufficient for strong adhesion of hMSCs to endothelial cells.
- Add DiI-labeled hMSCs to 80%-90% confluent monolayers of HUVECs and HMVECs-L in 12-well plates and incubate cells at 37 °C and 5% CO2.
- Incubate hMSCs which have been treated with blocking antibodies against integrin β1 at 4 μg/ml, integrin α5 at 10 μg/ml, integrin αVβ3 at 5 μg/ml, or control IgG at 10 μg/ml for 30 min at 37 °C.
- Wash samples once with PBS.
- Re-suspend samples with fresh growth medium and incubate for 30 min at 37 °C.
- Add cells to 80%-90% confluent monolayers of HUVECs or HMVECs-L in 12-well plates.
- Incubate culture seeded with leucocytes for 1 h, and culture seeded with hMSCs for 30 min.
- Wash the cultures twice with HBSS to remove non-adherent cells.
- Count the non-adherent cells with a hemocytometer.
- Fix the adherent cells with 1% PFA.
- Acquire images of adherent cells under a fluorescence microscope (200x), and 10 fields per well were imaged (Figure 1).
- Count the number of cells per field. Six duplicate wells are used for each condition.
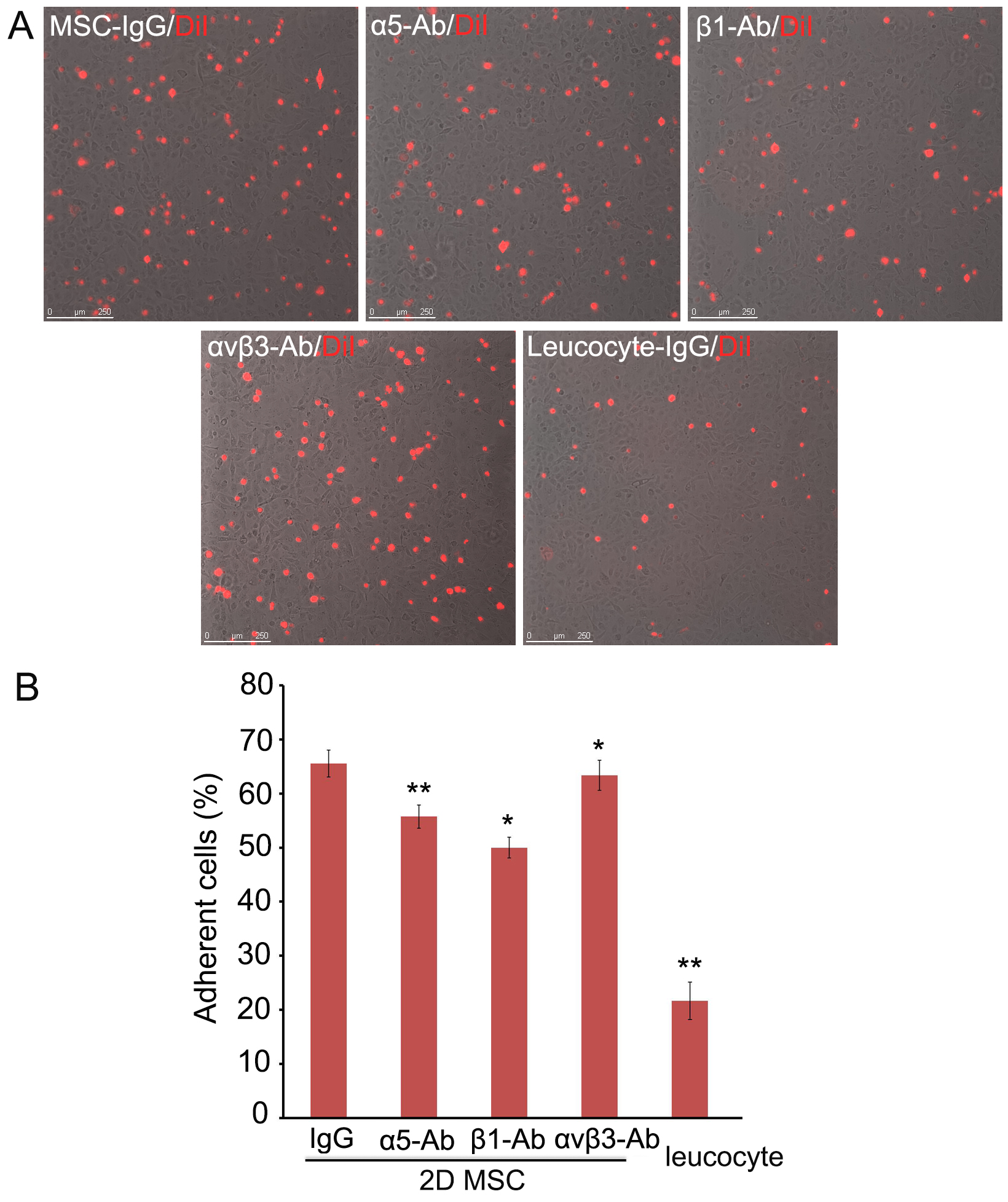
Figure 1. Adhesion of hMSCs to HMVEC-L. A. DiI-labeled single hMSCs derived from monolayers (2D) were pre-incubated with blocking antibodies against integrin α5, integrin β1, or integrin αVβ3, seeded on HMVECs-L monolayers and incubated for 30 min. hMSCs and leucocytes pre-incubated with isotype IgG were used as controls. Scale bars = 250 µm. B. The non-adherent cells were removed and counted, and the adherent DiI-hMSCs were photographed. Six replicate wells were used for each condition, and the experiment was repeated three times, * P < 0.05; ** P < 0.01. Abbreviation: MSC, mesenchymal stem cells.
- Culture HUVECs and HMVECs-L in 12-well plates with endothelial growth medium (EGM)-2 and EGMTM-2 MV BulletKitTM Medium, respectively, to confluence. The confluence density is approximate percentage of 80%-90%. Cells were treated with or without of 10 ng/ml TNF-α for 24 h immediately before the addition of hMSCs or leucocytes. TNF-α treatment, which is able to activate endothelial cells (Ko et al., 2009), increased the attachment of hMSCs and leucocytes to endothelial cells.
Data analysis
- Adherent cells (%) = (number of attached cells)/[total cell number] x 100%.
- The lowest concentrations of the blocking antibodies that achieved maximum inhibition to hMSCs attachment were chosen for experiments: integrin β1 at 4 μg/ml, integrin α5 at 10 μg/ml, and integrin αVβ3 at 5 μg/ml.
Notes
- Incubation for 30 min was the best time point for detection of hMSCs attachment to endothelial cells. Shorter incubation reduced the number of hMSCs in attachment, but longer incubation did not increase the number of hMSCs attached to endothelial cells.
- Pre-incubation with blocking antibodies against integrin β1, integrin α5, or integrin αVβ3 significantly decreased the number of hMSCs attached to the endothelial cells (P < 0.05), with integrin β1 blockade resulting in the most evident reduction (by ~40%).
- The non-adherent cells were analyzed using Dead Cell Apoptosis Kit by flow cytometry. At the above concentrations, blockade of integrin β1, but not integrin α5 nor integrin αVβ3 caused a modest increase of hMSCs apoptosis (2-3%) (P < 0.05).
- Our in vitro attachment experiments showed that functional blockade of these integrins in hMSCs significantly reduced their attachment to endothelial monolayers, but there were still more hMSCs attached to endothelial cells than leucocytes did. These results suggest that more adhesive molecules may be involved in hMSCs attachment to endothelial cells (Schenkel et al., 2004; Wang et al., 2015).
- We detected fibronectin, the major ligand of integrin α5β1, and vitronectin, the major ligand of integrin αVβ3, on cultured HMVECs-L and the normal endothelium in lungs.
Recipes
- Human leucocytes
Note: Leucocytes were isolated from the whole peripheral blood using Ficoll-paque Plus solution.- Place fresh blood into 15- or 50-ml conical centrifuge tubes
- Use a sterile pipet to add an equal volume of room-temperature PBS and mix well
- Slowly pipette the Ficoll-paque Plus solution by placing the tip of the pipette at the bottom of the sample tube containing blood/PBS mixture. Use 5 ml Ficoll-paque solution per 10 ml blood/PBS mixture
- Centrifuge for 30 min at 400 x g, 4 °C
- Use a sterile pipet, remove the upper layer that contains the plasma and most of the platelets
- Use another pipet to transfer the mononuclear cell layer to another centrifuge tube
- Wash cells by adding excess HBSS (~3 times the volume of the mononuclear cell layer) and centrifuge for 5 min at 400 x g (1,300 rpm), 4 °C
- Remove supernatant, re-suspend cells in HBSS, and repeat the wash once to remove most of the platelets
- Place fresh blood into 15- or 50-ml conical centrifuge tubes
Acknowledgments
This protocol was adapted from previously published papers (Luu et al., 2013; Wang et al., 2015; Wu et al., 2006). This work was supported by grants from the National Natural Science Foundation of China Natural Science Foundation of China (Nos. 31371404, 31571429), Natural Science Foundation of Guangdong (2015A030311041), and Shenzhen Science and Technology Innovation Committee (JCY20160301150838144).
Disclosure of potential conflicts of interest: The authors indicate no potential conflicts of interest.
References
- Bartosh, T. J., Ylostalo, J. H., Mohammadipoor, A., Bazhanov, N., Coble, K., Claypool, K., Lee, R. H., Choi, H. and Prockop, D. J. (2010). Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A 107(31): 13724-13729.
- Boyum, A. (1976). Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol Suppl 5: 9-15.
- Chacko, B. K., Kramer, P. A., Ravi, S., Johnson, M. S., Hardy, R. W., Ballinger, S. W. and Darley-Usmar, V. M. (2013). Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest 93(6): 690-700.
- Guo, L., Zhou, Y., Wang, S. and Wu, Y. (2014). Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) spheroids. J Cell Mol Med 18(10): 2009-2019.
- Ip, J. E., Wu, Y., Huang, J., Zhang, L., Pratt, R. E. and Dzau, V. J. (2007). Mesenchymal stem cells use integrin β1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell 18(8): 2873-2882.
- Ko, I. K., Kean, T. J. and Dennis, J. E. (2009). Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials 30(22): 3702-3710.
- Lee, R. H., Pulin, A. A., Seo, M. J., Kota, D. J., Ylostalo, J., Larson, B. L., Semprun-Prieto, L., Delafontaine, P. and Prockop, D. J. (2009). Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5(1): 54-63.
- Luu, N. T., McGettrick, H. M., Buckley, C. D., Newsome, P. N., Rainger, G. E., Frampton, J. and Nash, G. B. (2013). Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment. Stem Cells 31(12): 2690-2702.
- Salem, H. K. and Thiemermann, C. (2010). Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28(3): 585-596.
- Schenkel, A. R., Mamdouh, Z. and Muller, W. A. (2004). Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol 5(4): 393-400.
- Toma, C., Wagner, W. R., Bowry, S., Schwartz, A. and Villanueva, F. (2009). Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res 104(3): 398-402.
- Wang, S., Guo, L., Ge, J., Yu, L., Cai, T., Tian, R., Jiang, Y., Zhao, R. and Wu, Y. (2015). Excess integrins cause lung entrapment of mesenchymal stem cells. Stem Cells 33(11): 3315-3326.
- Wu, Y., Ip, J. E., Huang, J., Zhang, L., Matsushita, K., Liew, C. C., Pratt, R. E. and Dzau, V. J. (2006). Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res 99(3): 315-322.
- Wu, Y. and Zhao, R. C. (2012). The role of chemokines in mesenchymal stem cell homing to myocardium. Stem Cell Rev 8(1): 243-250.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Wang, S., Madsen, C. D. and Wu, Y. (2018). Measurement of Mesenchymal Stem Cells Attachment to Endothelial Cells. Bio-protocol 8(6): e2776. DOI: 10.21769/BioProtoc.2776.
Category
Stem Cell > Adult stem cell > Mesenchymal stem cell
Cell Biology > Cell-based analysis > Cell adhesion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link