- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Mono Sodium Urate Crystal-induced Peritonitis for in vivo Assessment of Inflammasome Activation
Published: Vol 8, Iss 5, Mar 5, 2018 DOI: 10.21769/BioProtoc.2754 Views: 9935
Reviewed by: Jia LiLokesh KalekarKathrin Sutter

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2542 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views
Abstract
Due to its particulate material, mono-sodium urate (MSU) crystals are potent activators of the NOD-like receptor NLRP3. Upon activation, NLRP3 induces the formation of inflammasome complexes, which lead to the production and release of mature IL-1β. Bioactive IL-1β is a potent activator of innate immune responses and promotes recruitment of inflammatory cells, including neutrophils from the blood into damaged/inflamed tissues. This protocol describes a method to study in vivo inflammasome activation via intraperitoneal injection of MSU crystals. MSU-injection results in a drastic increase of intraperitoneal IL-1β levels, promoting neutrophil infiltration. Early-stage neutrophil numbers correlate with the amount of released IL-1β and can be used as a read-out for the extent of in vivo inflammasome activation. In addition, this protocol might also be used as a sterile peritonitis model, to investigate mechanisms of neutrophil recruitment to the peritoneum, or as a means to obtain large numbers of in vivo activated neutrophils.
Keywords: (sterile) PeritonitisBackground
Innate immune cells recognize pathogens through a set of pattern recognition receptors (PRR), which bind to evolutionarily conserved structures on the pathogen surfaces or through ligation of other danger-associated molecular patterns. One family of these receptors are the NOD-like receptors (NLR), which react to the intracellular presence of invading pathogens and/or intracellular danger signals (Meylan et al., 2006). Several PRR, including some NLRs are capable of inducing the formation of so-called inflammasome complexes, which mediate the proteolytic activation of pro-IL-1β, pro-IL-18, and other IL-1 family cytokines (Martinon et al., 2002). Due to the potent pro-inflammatory nature of IL-1β and IL-18, inflammasome activation is a highly regulated, two-step process, involving limited transcription of pro-IL-1β/pro-IL-18, and highly regulated activation of inflammasome receptors (Martinon et al., 2009). NLRP3, one of the most studied inflammasome receptors, responds to a great variety of intracellular danger-associated molecular patterns, including bacterial cell wall components (Martinon et al., 2004), damaged mitochondria (Zhou et al., 2011), and particulate materials (Martinon et al., 2006). Due to their particulate structure, mono sodium urate (MSU) crystals are very potent NLRP3 activators (Martinon et al., 2006), which are widely used for in vitro studies of NLRP3 activation.
In addition to its use for in vitro experiments, MSU can also be used to study the in vivo relevance of inflammasome activation. Here, we described an MSU-induced peritonitis model to easily and quickly study the in vivo relevance and extent NLRP3-inflammasome activation, e.g., upon genetic deletion of proteins that are involved in NLRP3 activation (Chen et al., 2006, Spalinger et al., 2016). In the MSU-induced peritonitis, the first wave of infiltrating immune cells consists mainly of neutrophils, and in the early phase of peritonitis, the number of infiltrating neutrophils correlates with the extent of inflammasome activation and with the production of mature IL-1β (Chen et al., 2006; Spalinger et al., 2016).
Materials and Reagents
- Pipette tips
- Insulin syringes (BD, catalog number: 324826 )
- 5 ml syringes (BD, catalog number: 302187 )
- 25 G needles (Terumo, catalog number: GS-351 )
- 50 ml tubes (Corning, Falcon®, catalog number: 352070 )
- FACS tubes with lid (Corning, Falcon®, catalog number: 352058 )
- Mice: C57BL/6 adult females (THE JACKSON LABORATORY, catalog number: 000664 )
Note: This protocol has been developed for C57BL/6 mice. For other mouse strains, MSU concentration and optimal time until peritoneal lavage should be titrated. - Mono-sodium urate (MSU) crystals (InvivoGen, catalog number: tlrl-msu )
- Fluorescent antibody against Ly6G (for example, AlexaFluor647 anti-Ly6G [clone 1A8], BioLegend, catalog number: 127609 )
- Fluorescent antibody against Ly6B.2 (also known as 7/4 antigen; for example Fitc anti-Ly6B.2 [clone REA115], Miltenyi Biotec, catalog number: 130-103-318 )
- Fluorescent antibody against CD3ε (for example, PE-CF594 anti-CD3ε [clone 145-2C11], BD, BD Biosciences, catalog number: 562286 )
- Fluorescent antibody against CD45 (for example, Pacific Blue anti-CD45 [clone 30F11], BioLegend, catalog number: 103126 )
- Live-dead discriminator (for example Zombie NIR Fixable Viability Kit, BioLegend, catalog number: 423105 )
- Mouse IL-1 beta/IL-1F2 DuoSet ELISA kit (R&D Systems, catalog number: DY401 )
- Substrate Reagent Pack (R&D Systems, catalog number: DY999 ) for ELISA
- Dulbecco’s modified PBS (Sigma-Aldrich, catalog number: D8537-500ML )
- Fetal calf serum (for example, PAN-Biotech, catalog number: P40-47100 )
- FACS buffer (see Recipes)
Equipment
- Pipettes
- Dissection tools (sharp scissors and forceps)
- Neubauer cell counting chamber or automated cell counter
- Refrigerated benchtop centrifuge
- Flow cytometer
- ELISA plate reader
Procedure
The whole procedure is summarized in Figure 1. All animal experiments were performed in accordance to Swiss animal welfare legislation.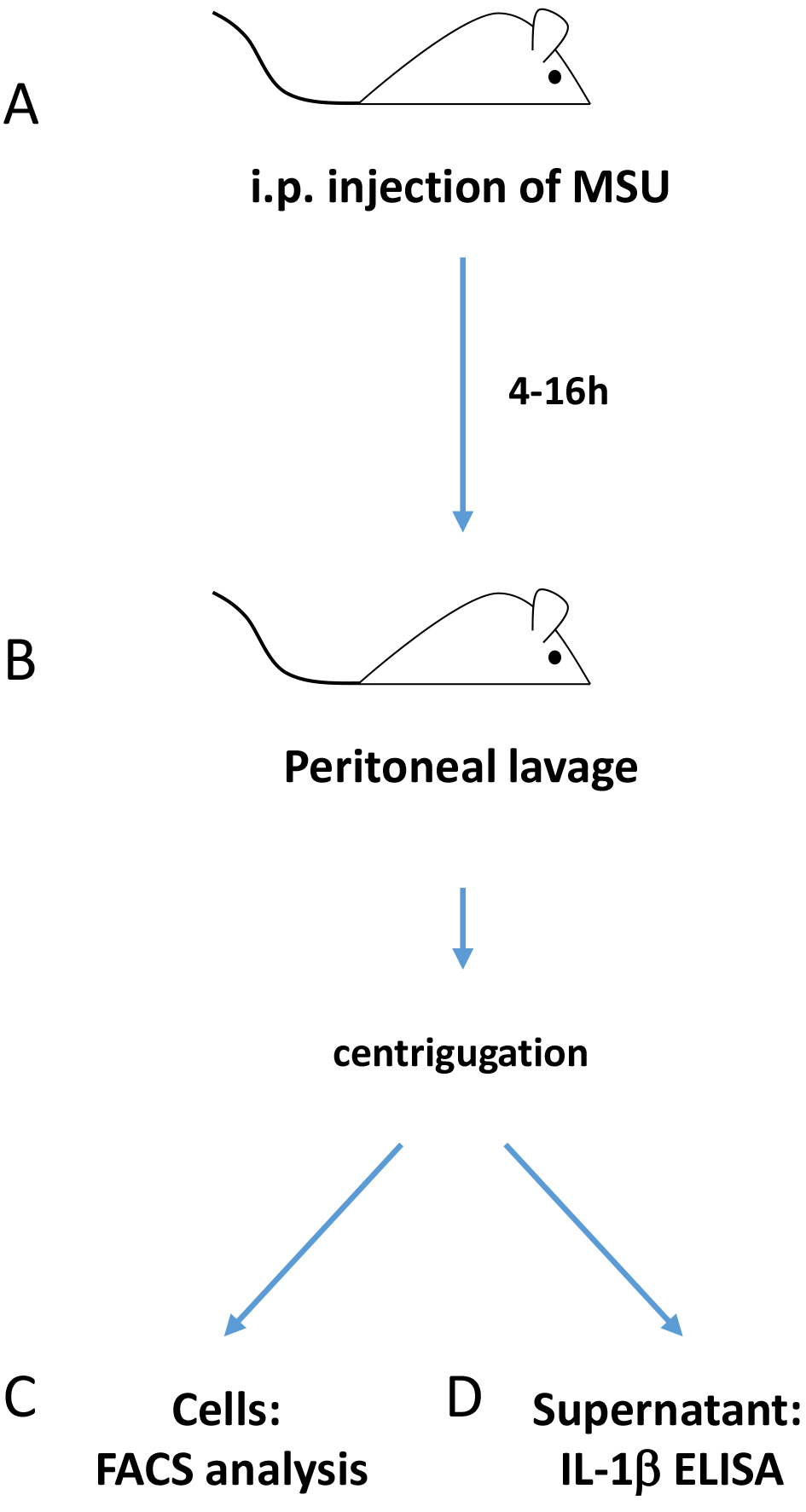
Figure 1. Overview of the procedure. The scheme summarizes the principal steps of this protocol for assessing in vivo inflammasome activation via peritoneal injection of MSU.
- MSU injection into the peritoneal cavity (see Video 1, which shows how to perform intraperitoneal injections)
Video 1. Procedure to perform intra-peritoneal injections- Prepare MSU suspension: add 0.5 ml of sterile PBS to one vial of MSU crystals (5 mg) and vortex thoroughly (> 5 min for initial resuspension, later vortex for 1 min is sufficient) to obtain a suspension of 10 mg/ml MSU.
Note: MSU crystals do NOT dissolve in PBS and are injected as a suspension. Do not centrifuge; vortex for 1 min prior to use. - Mark each mouse by ear punch or toe clipping as per local animal welfare legislation and animal experimental license.
Note: Since the experiment lasts max 16 h, mice can also be marked transiently using a waterproof bench marker. - Inject 180 μl of MSU suspension or 180 μl sterile PBS (control mice) into the peritoneal cavity using an insulin syringe:
- Vortex the MSU suspension before drawing into the syringe.
- Hold the mouse slightly inclined towards its head.
- Insert the needle at a 30°-45° angle. Make sure that you are in the peritoneal cavity and slowly inject the suspension.
- Vortex the MSU suspension before drawing into the syringe.
- Prepare MSU suspension: add 0.5 ml of sterile PBS to one vial of MSU crystals (5 mg) and vortex thoroughly (> 5 min for initial resuspension, later vortex for 1 min is sufficient) to obtain a suspension of 10 mg/ml MSU.
- Peritoneal lavage and collection of cells
Perform peritoneal lavage as shown in Video 2 at the desired time-point of analysis (typically 4 h, 8 h, and 16 h after MSU injection):
Video 2. Procedure to perform a peritoneal lavage- Euthanize the mouse by cervical dislocation or CO2-asphyxiation.
Note: Process one mouse after each other, the mice should not become stiff before the cell harvest is complete. Take care that no blood vessels bleed into the peritoneal cavity when euthanizing by cervical dislocation. - Open the skin of the belly carefully without damaging the peritoneum.
- Inject 5 ml PBS into the peritoneal cavity using a 25 G needle.
- Shake the mouse for 2-3 min.
- Aspirate the PBS from the peritoneal cavity using the same syringe, transfer into a 50 ml conical tube, measure the amount of recovered PBS.
Note: An experienced experimenter recovers approx. 4 ml of the injected PBS. - Determine the cell concentration per ml using a Neubauer counting chamber or an automated cell counter.
Note: Red cell lysis is not required, but make sure not to count red blood cells, debris, or dead cells.
- Euthanize the mouse by cervical dislocation or CO2-asphyxiation.
- Flow cytometry to characterize cell infiltrate
- Take 1 x 106 cells from each lavage, spin down, transfer to FACS tube.
- Resuspend the cells in 50 μl PBS containing:
- AlexaFluor anti-Ly6G antibody, 1 μg/ml;
- Fitc anti-Ly6B.2 antibody, 1 μg/ml;
- PE-CF594 anti-CD3ε antibody, 0.5 μg/ml;
- Pacific Blue anti-CD45 antibody, 0.5 μg/ml;
- Zombie-NIR Live-dead discriminator (dilute 1:800).
- AlexaFluor anti-Ly6G antibody, 1 μg/ml;
- Incubate for 20 min on ice in the dark.
- Add 100 μl FACS buffer to each tube.
- Spin down at 350 x g for 5 min.
- Remove supernatant and wash once more with 100 μl FACS buffer.
- Resuspend in 100 μl FACS buffer and proceed to analysis at Flow cytometer. Figure 2 shows typical results and gating strategy used to identify live, single, CD45+ cells.
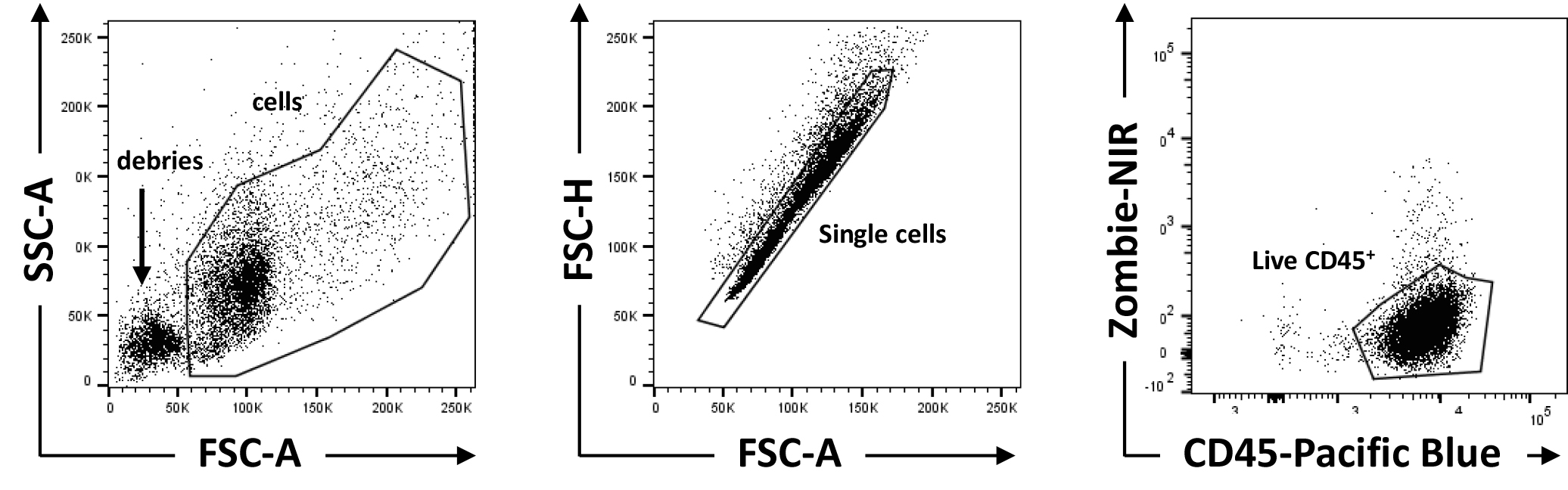
Figure 2. Gating strategy. Gating strategy to exclude debris, doublets, and dead cells from the analysis. - CD45+, Ly6G+, Ly6B.2+ cells are neutrophils. Figure 3A shows typical flow cytometry dot plots when gated on single, live CD45+ cells.
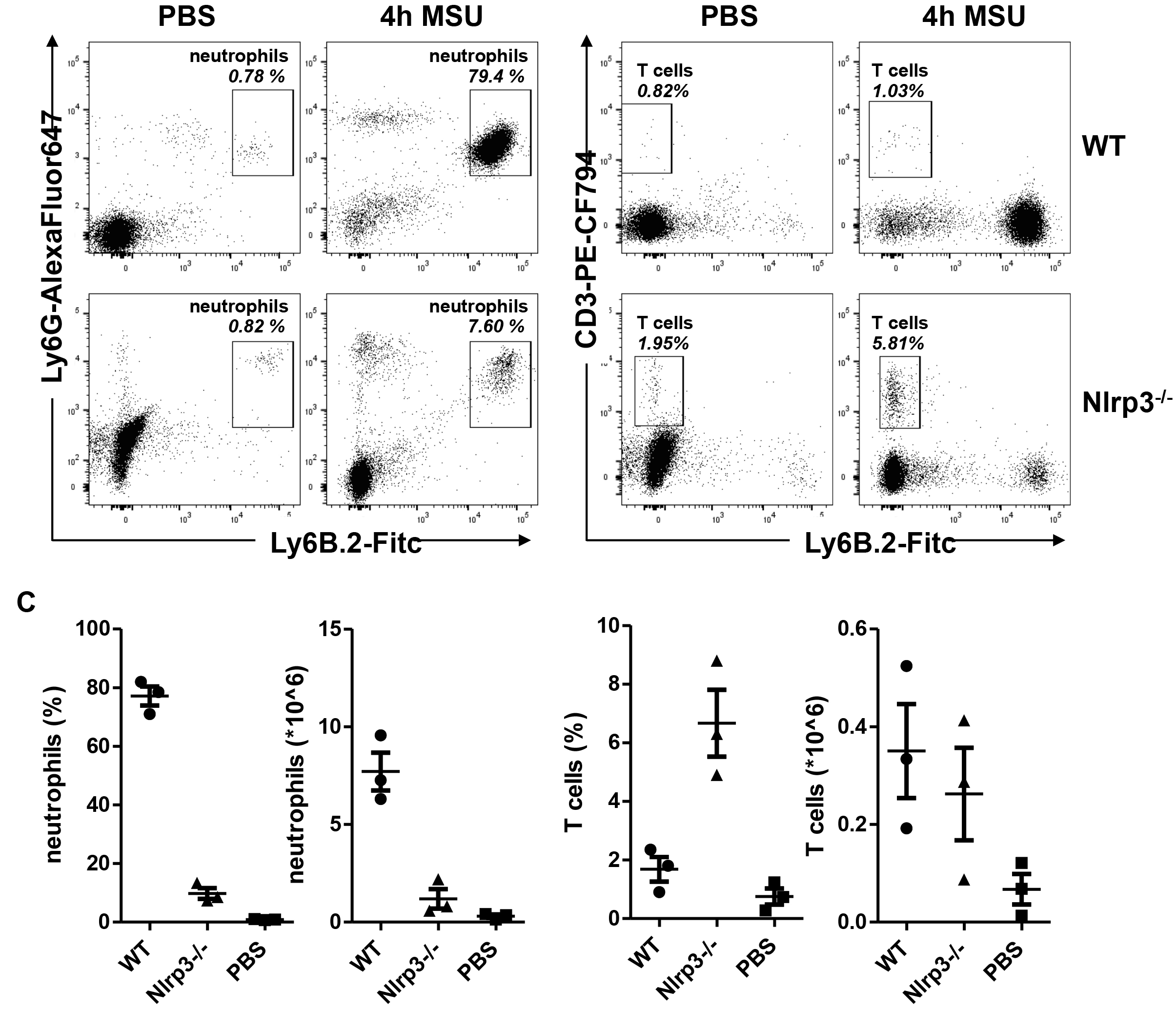
Figure 3. Representative data from flow cytometry. A. Representative flow cytometry plots of peritoneal cells collected 4 h after intraperitoneal injection of MSU. B. Representative results of relative and absolute numbers of infiltrating neutrophils (left) and T cells (right). Each point represents one mouse.
- Take 1 x 106 cells from each lavage, spin down, transfer to FACS tube.
- Quantification of IL-1β in peritoneal lavage
- Take 0.5 ml of peritoneal lavage from Step B6.
- Spin the cells down, use supernatant for the analysis.
Note: IL-1β levels can be rather low; do not dilute the supernatant for ELISA analysis. - Perform IL-1β ELISA according to the manufacturer’s instructions.
Figure 4 shows typical results of IL-1β ELISA on peritoneal lavages.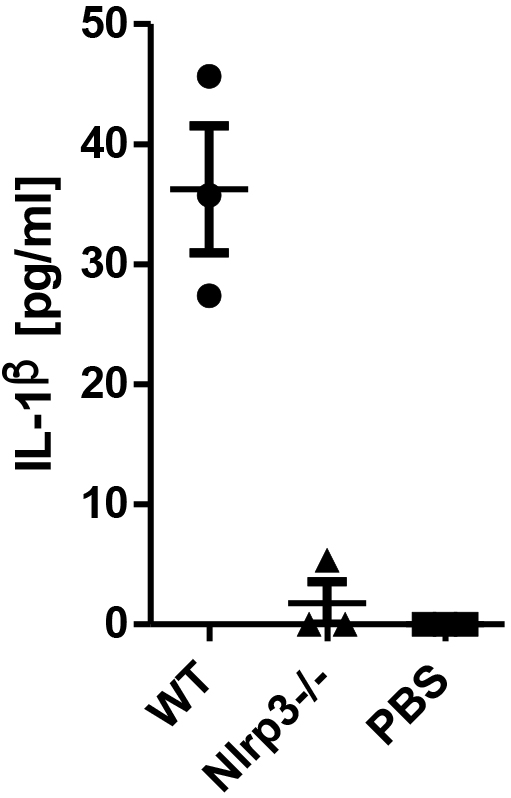
Figure 4. Representative data from ELISA measurement. IL-1β ELISA from peritoneal lavage collected from WT and NLRP3-/- mice 8 h after intra-peritoneal MSU injection. For the lavage, 3 ml of PBS was injected into the peritoneal cavity. Each dot represents one mouse.
- Take 0.5 ml of peritoneal lavage from Step B6.
Data analysis
The number of infiltrating neutrophils is calculated as follows:
- Calculate the absolute number of neutrophils: cell concentration (cell count per ml) obtained in Step B6 x 5 = total number of cells.
- Calculate the number of infiltrating neutrophils: the total number of cells x frequency of Ly6G+, Ly6C+ cells of single, live, CD45+ cells = absolute number of infiltrating neutrophils.
Accordingly, the calculation would be: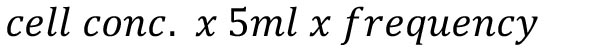
With cell conc. = concentration of cells obtained in Step B6; and frequency = proportion of cells in the Lys6G+/Ly6B.2+ gate (see Figure 3A, left panels).
If desired, the same approach is used to determine absolute numbers of T cells in the peritoneum, using the frequency of CD3+ cells within live, single, CD45+ cells. Figure 3B shows typical results obtained with this method.
Notes
- All animal experiments should be carried out according to local animal welfare legislation.
- The used concentration of MSU was tested for C57BL/6 mice and may require titration if other mouse strains are used.
- As a measure for inflammasome activation, 4 h and/or 8 h are typical time-points of analysis. However, the experiment can also be performed for up to 24 h. However, at later time-points, secondary factors influence the results and number of infiltrating cells might no longer correlate directly with inflammasome activity.
- IL-1β levels in the peritoneal lavage can be rather low. If IL-1β measurement is the primary read-out, lavage can be performed with as little as 2.5 to 3 ml PBS. However, this results in reduced numbers of recovered cells, since only approx. 2.0 to 2.5 ml of the injected PBS can be recovered.
- We typically use Nlrp3-/- mice as a negative control for MSU-induced peritonitis, since these mice are defective for the inflammasome receptor primarily involved in recognizing MSU.
Recipes
- FACS buffer
Supplement Dulbecco’s PBS with 2% fetal calf serum
Keep sterile and store at 4 °C for up to 2 months
Acknowledgments
This protocol was first described by Chen et al. (2006) and was further developed for a study by our group (Spalinger et al., 2016), which was supported by the Swiss National Science Foundation (314730-146204; CRSII3_154488/1; 310030-120312), the Zürcher Universitäts-Verein, and the Swiss Philanthropy Foundation. The authors declare no conflicts of interest or competing financial interests.
References
- Chen, C. J., Shi, Y., Hearn, A., Fitzgerald, K., Golenbock, D., Reed, G., Akira, S. and Rock, K. L. (2006). MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest 116(8): 2262-2271.
- Martinon, F., Agostini, L., Meylan, E. and Tschopp, J. (2004). Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol 14(21): 1929-1934.
- Martinon, F., Burns, K. and Tschopp, J. (2002). The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10(2): 417-426.
- Martinon, F., Mayor, A. and Tschopp, J. (2009). The inflammasomes: guardians of the body. Annu Rev Immunol 27: 229-265.
- Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. and Tschopp, J. (2006). Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440(7081): 237-241.
- Meylan, E., Tschopp, J. and Karin, M. (2006). Intracellular pattern recognition receptors in the host response. Nature 442(7098): 39-44.
- Spalinger, M. R., Kasper, S., Gottier, C., Lang, S., Atrott, K., Vavricka, S. R., Scharl, S., Gutte, P. M., Grutter, M. G., Beer, H. D., Contassot, E., Chan, A. C., Dai, X., Rawlings, D. J., Mair, F., Becher, B., Falk, W., Fried, M., Rogler, G. and Scharl, M. (2016). NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J Clin Invest 126(5): 1783-1800.
- Zhou, R., Yazdi, A. S., Menu, P. and Tschopp, J. (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329): 221-225.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Spalinger, M. R. and Scharl, M. (2018). Mono Sodium Urate Crystal-induced Peritonitis for in vivo Assessment of Inflammasome Activation. Bio-protocol 8(5): e2754. DOI: 10.21769/BioProtoc.2754.
Category
Immunology > Animal model > Mouse
Immunology > Inflammatory disorder > Inflammasome
Cell Biology > Cell-based analysis > Inflammatory response
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link