- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Large Scale Field Inoculation and Scoring of Maize Southern Leaf Blight and Other Maize Foliar Fungal Diseases
Published: Vol 8, Iss 5, Mar 5, 2018 DOI: 10.21769/BioProtoc.2745 Views: 9238
Reviewed by: Zhibing LaiGuan-Feng WangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Rice Ragged Stunt Virus Propagation and Infection on Rice Plants
Chao Zhang [...] Jianguo Wu
Oct 20, 2018 6497 Views

Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Piao Yang [...] Ye Xia
Oct 20, 2023 2764 Views
Abstract
Field-grown maize is inoculated with Cochliobolus heterostrophus, causal agent of southern leaf blight disease, by dropping sorghum grains infested with the fungus into the whorl of each maize plant at an early stage of growth. The initial lesions produce secondary inoculum that is dispersed by wind and rain, causing multiple cycles of infection that assures a high uniform disease pressure over the entire field by the time of disease scoring, which occurs after anthesis. This method, with slight modifications, can also be used to study the maize fungal diseases northern leaf blight (caused by Exserohilum turcicum) and gray leaf spot (Cercospora zeae-maydis).
Keywords: MaizeBackground
Southern leaf blight (SLB), caused by Cochliobolus heterostrophus (Drechs.) Drechs. [anamorph = Bipolaris maydis (Nisikado) Shoemaker], is a widespread maize disease which causes significant yield losses in hot, humid tropical and sub-tropical regions, such as the southeastern USA, parts of India, Africa, Latin America and Southern Europe. In 1970-71 an SLB epidemic caused by C. heterostrophus race T infecting hybrids carrying Texas male-sterile cytoplasm (cms-T) caused an estimated 15% loss in total maize production in the US (Ullstrup, 1972). After the 1970 epidemic, cms-T maize was replaced by race T-resistant, normal cytoplasm maize.
Currently, race O is the predominant cause of SLB in the US and worldwide (Wang et al., 2017). SLB resistance to C. heterostrophus race O is quantitatively inherited with primarily additive or partially dominant gene action (Holley and Goodman, 1989). Under experimental conditions, yield losses due to infection with C. heterostrophus race O as high as 46% have been observed (Fisher et al., 1976; Byrnes and Pataky, 1989). However, losses in commercial production are generally much less severe (Mueller et al., 2016).
This approach to inoculation and rating is based on methodology developed by Carson et al. (2004), though similar methods had been used in numerous previous studies (e.g., Fisher et al., 1976). We have used it in a number of studies to screen germplasm for SLB resistance and to elucidate its genetic basis (Balint-Kurti et al., 2006; 2007 and 2008b; Zwonitzer et al., 2009 and 2010; Kump et al., 2011; Negeri et al., 2011; Belcher et al., 2012; Santa-Cruz et al., 2014; Yang et al., 2017). We have also used an essentially identical method to assess resistance to two other foliar fungal diseases; Gray leaf spot caused by Cercospora zeae-maydis (e.g., Balint-Kurti et al., 2008a) and northern leaf blight (NLB) caused by Exserohilum turcicum (e.g., Balint-Kurti et al., 2010; Chung et al., 2010; Zwonitzer et al., 2010). This method has provided reliable data with high correlations between replications and environments.
Materials and Reagents
- Petri dishes, 100 x 15 mm (Genesee Scientific, catalog number: 32-107G )
- Micro-spatula (VWR, catalog number: 82027-518 )
- Parafilm M (Bemis, catalog number: PM999 )
- 50-ml conical tubes (Corning, Falcon®, catalog number: 352070 )
- 15-ml sterile tube
- Garbage bag large enough to line cooler
- Newspapers
- Gloves
- Identi-plug foam plugs (Jaece Industries, catalog number: L800-E )
- Aluminum foil
- Small metal beads (Ballistic Products, #4 shot SHZ04 or similar)
- Isolates of Cochliobolus heterostrophus frozen in 50% glycerol
- Sorghum grain (wheat or barley may also be used)
Note: The sorghum should not be treated with any chemicals or fungicides. Sorghum intended for birdseed, also called milo, is ideal. - Difco Potato Dextrose Agar (PDA) media (BD, DifcoTM, catalog number: 213400 )
- A small quantity of 70% ethanol in a glass container with a lid
- Tween-20
- V8 juice
- Agar
- CaCO3
- V8-agar medium (see Recipes)
Equipment
- Laminar flow workbench (NuAire, model: AireGardTM ES NU-301 , catalog number: 301-630)
- Incubator (Percival Scientific, model: I-35LL )
- Tongs or large tweezers
- Scalpel (EISCO, catalog number: BIO182A )
- 1 L Erlenmeyer flasks (Corning, PYREX®, catalog number: 5100-1L )
- Autoclave
- Alcohol lamp (such as C&A Scientific, catalog number: 97-5313 ) filled with ethanol
- Plastic buckets
- Ventilated trays (Buckhorn, catalog number: BT28220522 )
- Oscillating fan (Air King, catalog number: 9119 )
- Cooler
- Pails
Procedure
- Propagating fungal cultures on potato dextrose agar (the work should be performed inside a laminar flow hood)
- Make PDA plates, preparing media and autoclaving according to package instructions. After autoclaving, the molten PDA should be allowed to cool to about 60 °C before pouring approximately 18 to 20 ml into each Petri dish.
- Flame-sterilize the micro-spatula and use it to scoop a small chunk–50 to 100 µl–of frozen C. heterostrophus fungal stock onto a plate; repeat for each desired C. heterostrophus isolate. Seal the plates with Parafilm and place them into an incubator at constant 25 °C, 12-h light/dark. Allow to grow for 10 to 14 days.
- Propagate the fungal cultures: To produce several plates from the initial plate, use a flame-sterilized scalpel to cut a 1-cm square from the leading edge of a colony and dab it over the surface of a fresh plate. Incubate as before (Step A2). These secondary plates will be used to inoculate the grain.
- If desired, cultures can be transferred again onto fresh PDA plates for several more generations. Plates should be used at least 10 to 14 days after inoculation, but older plates can be used. We have used plates as old as 3 months. Fungal pathogens are known to sometimes become less aggressive pathogens after prolonged growth and repeated transfer on plates. While we have not observed this phenomenon with C. heterostrophus, we start a fresh culture from the frozen stock to produce inoculum each season.
- Make PDA plates, preparing media and autoclaving according to package instructions. After autoclaving, the molten PDA should be allowed to cool to about 60 °C before pouring approximately 18 to 20 ml into each Petri dish.
- Propagating fungal cultures on grain
- Begin preparation of the grain three or four days before inoculating with fungal cultures. Fill plastic buckets about half full with sorghum (or barley or wheat), then add lukewarm water to three-quarters full. Allow the grain to soak for three days.
- Drain the water away by holding a screen against the edge of the bucket to catch debris and pouring the water off. Scoop the grain into 1-L flasks, filling to 700 ml. As each flask is filled with grain, add water to the flask, swirl to rinse the grain, and strain the water away through a screen.
- Cap each flask with an Identi-plug and cover the top with a square of foil. Autoclave for 1 h at 121 °C. Allow to cool.
- Prepare sterile tubes for production of fungal suspension: Into each 50-ml conical tube, add about 4 metal beads and 35 ml water. Autoclave for 15 min at 121 °C. Allow to cool.
- Prepare fungal suspension using the sterile tubes and fungal cultures grown on PDA plates: In laminar flow hood using a flame-sterilized scalpel, cut the fungal mat and underlying media into thin strips. Divide the material from one plate into two tubes (Figure 1). Cap the tubes tightly and shake vigorously to further break up the fungal culture. This will produce a slurry containing a suspension of fungal spores and mycelial fragments.
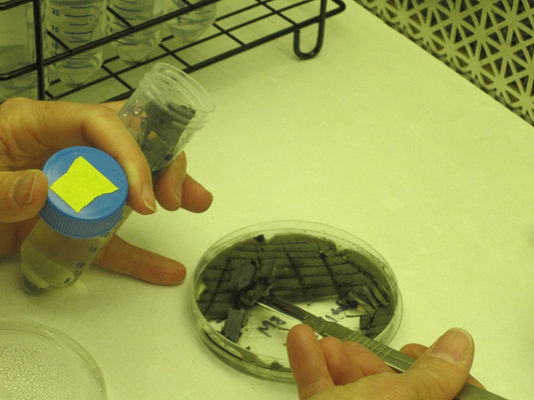
Figure 1. Making fungal suspension - Inoculate the grain: In the laminar flow hood, one tube of fungal suspension can be dispersed into three or four flasks of sterilized grain. Collect the flasks and the tube of fungal suspension. Shake the flasks to loosen grain. Remove the foil caps. Flame-sterilize the large forceps, shake the fungal suspension and remove the cap, then work quickly to remove a foam stopper with the forceps, pour in 10-15 ml of fungal suspension, and replace the foam stopper for each flask (Figure 2). Replace the foil caps. Gently shake each flask to distribute the fungus.

Figure 2. Pouring the fungal suspension into sterile sorghum grain - Incubate on a lab bench at room temperature (Figure 3) for about 10 days or until grains are evenly covered in fungus (Figure 4), shaking the flasks every 2 to 3 days to distribute the fungus and allow for even growth. Vigorous shaking is sometimes required to break up clumps.

Figure 3. Hyphae growing from PDA fragment 1 day post-inoculation
Figure 4. A mature sorghum grain culture - Dry the grain inoculum: Empty the inoculum from the flasks into buckets lined with plastic bags. A sterile spatula can be used to break up large clumps. Transport to a dry, covered location such as a garage or barn. Line ventilated trays with newspaper and spread the grain in a layer no deeper than 3 cm. Allow the trays of inoculum to dry, using a fan for air circulation; stir and break up clumps with gloved hands every few days. It is important that the grain inoculum is dried rapidly as it is susceptible to molding. The drying process usually takes about two weeks.
- When the inoculum is completely dry, store in closed plastic bags at 2-7 °C and < 50% relative humidity. The inoculum may remain viable for several years if well-dried and kept cool.
- Begin preparation of the grain three or four days before inoculating with fungal cultures. Fill plastic buckets about half full with sorghum (or barley or wheat), then add lukewarm water to three-quarters full. Allow the grain to soak for three days.
- Inoculating maize
- The field experiment should be set up with one or two rows of border corn on all sides of the experimental block so that experimental plots do not experience ‘edge effects’. Disease symptoms are often slightly lower on the very edges of a field due, presumably, to lower disease pressure and humidity. We generally set up two or three replications of each experiment and try to physically separate the replications as much as possible so that if one replication is planted in a ‘bad’ part of the field, the other replication is unlikely to be in that same area. In general, most experiments are performed over two or three years or environments with two or three replications per environment/year.
- Field-grown maize plants should ideally be inoculated as early as possible after most plants in the field have formed a whorl. This is usually after about 5 to 6 weeks growth when the plants are at the 7 to 9 leaf stage, although when working with diverse lines, some will likely be larger and smaller. Some variation within the field is acceptable. If some plants are too small to inoculate that is also OK as they will be infected by the secondary inoculum that is generated.
- On the day of inoculation, combine the dry sorghum grain inoculum of the desired fungal isolates in a cooler. About 10 gallons of inoculum is used to inoculate about 3 acres of experiments or approximately 40,000 plants.
- If a large experiment (say more than 2 acres) is to be inoculated, it is best to assemble a team of several volunteers, say 1-2 people per acre, to inoculate it. This is because inoculation can take some time and is strenuous since there is a lot of bending involved. Give each volunteer a pail of inoculum and have them drop about ten to twenty grains of inoculum into the whorl of each maize plant (Figure 5, Video 1). Move across the field row by row until every row is inoculated. N.B. While the goal is to inoculate every plant with an equal amount of inoculum, this is not realistically achievable, nor is it essential. The goal of the initial inoculation is to generate secondary inoculum which will spread over the field creating a high, uniform disease pressure. For the same reason, it is not important that the same person inoculate a whole experiment.

Figure 5. Details of field inoculation. A. Applying inoculum in the field; B. Maize plants immediately after inoculation.Video 1. Video of field inoculation - The inoculum is ‘activated’ by moisture which initiates fungal growth. If overhead irrigation is available, it should be applied briefly. If not, rain or dew will collect in the whorl, allowing the fungus to grow and proliferate. Initial SLB symptoms should be apparent in 4-7 days (Figure 6), but disease severity should not be rated based on this early infection.

Figure 6. Maize plants 2 weeks after inoculation, showing disease symptoms where leaves were directly inoculated. Subsequent growth is initially free of symptoms, though symptoms will develop on these leaves later in the season.
- The field experiment should be set up with one or two rows of border corn on all sides of the experimental block so that experimental plots do not experience ‘edge effects’. Disease symptoms are often slightly lower on the very edges of a field due, presumably, to lower disease pressure and humidity. We generally set up two or three replications of each experiment and try to physically separate the replications as much as possible so that if one replication is planted in a ‘bad’ part of the field, the other replication is unlikely to be in that same area. In general, most experiments are performed over two or three years or environments with two or three replications per environment/year.
- Scoring disease
- In general, the initial symptoms of the disease arising from the primary inoculum are quite severe but the corn quickly outgrows these and much milder (or no) symptoms are apparent initially on subsequent growth. Symptoms then become more severe after flowering. Plants should be rated for disease soon after anthesis when an ear is apparent, and then one to three times more at approximately 10-14 day intervals. Disease is rated by observing the ear leaf and the leaf above, then rating the symptoms on a 1 to 9 scale where the maximum score of nine indicates no disease symptoms and the minimum score of one indicates complete death of the plant (Figure 7, reproduced from Kump et al. (2011)). Generally, several plants in each plot are inspected briefly and one representative score is recorded for each plot unless disease severity obviously differs from plant to plant. In general an experienced scorer can rate a plot in about 15 to 20 sec.
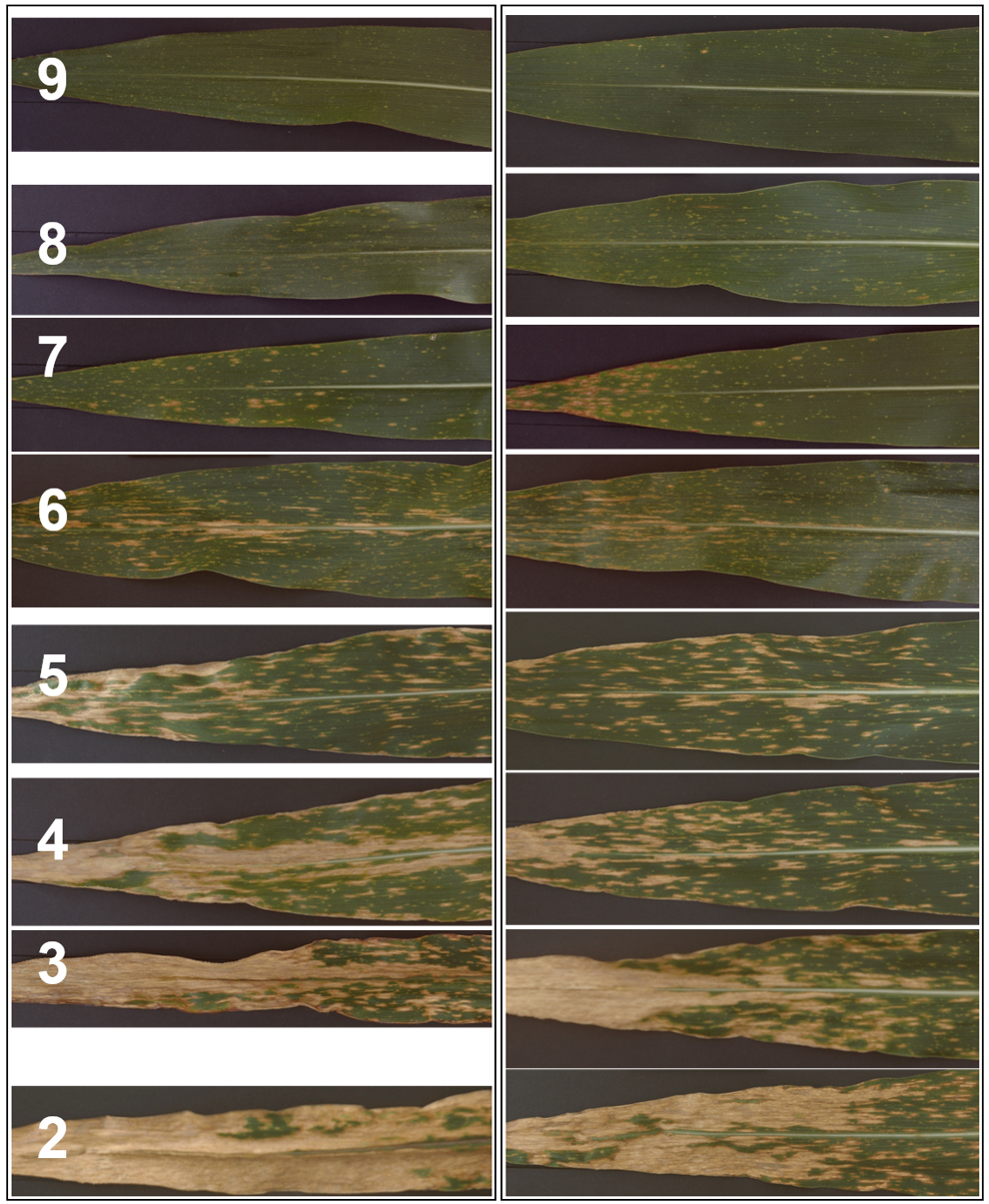
Figure 7. SLB Disease symptom scoring guide. SLB scoring rubric: 9-No evidence of leaf blight; 8-A few spots on the lower leaves; 7-A few spots on the ear leaf; 6-More spots on the ear leaf but the lesions don’t coalesce; 5-Lesions on the ear leaf have grown together, particularly at the tip of the leaf to give quite large necrotic areas; 4-Lesions on the leaf above the ear leaf have grown together too; 3-Leaf above the ear leaf almost completely dead; 2-Almost all tissue on the plant dead; 1-Everything brown (reproduced from Kump et al., 2011). - Once the plant has started to senesce such that necrosis due to senescence is visible on the ear leaf, then it is no longer possible to score disease accurately. In general, scoring is possible for about five weeks after the start of anthesis but unusually hot dry weather can decrease this period.
- In hotter environments, for instance in the southern US, leaves will curl up in the afternoons, making scoring difficult. In these cases, it is advantageous to try to start to score as early as possible in the morning before curling occurs.
- The Field Book app (Rife and Poland, 2014) on a tablet computer is useful for recording scores of large populations in the field.
- It is important that an entire experimental replication is rated on the same day by the same person since the disease progresses rapidly and scores may change from day to day. We have found that scores between different scorers are somewhat variable but that relative score differentials between plots do not vary significantly. This has been noted elsewhere (Poland and Nelson, 2011).
- In some cases, for various reasons, individual plants or rows or sets of rows may not grow as they should or may be damaged by insect or animal pests. In our experience, it is preferable to record the scores of such rows as missing data rather than to try to ascribe a score to a row or plant that may not be accurate. We have found that plants that do not produce a healthy ear with normal developing seed may give inaccurate scores since source-sink relationships in the plant significantly affect the development of symptoms. While in general, the disease pressure is uniform across the field, various factors such as soil type and local topography may cause the disease pressure to vary somewhat. Rows that are to be directly compared should therefore be planted as close to each other as possible.
- In general, the initial symptoms of the disease arising from the primary inoculum are quite severe but the corn quickly outgrows these and much milder (or no) symptoms are apparent initially on subsequent growth. Symptoms then become more severe after flowering. Plants should be rated for disease soon after anthesis when an ear is apparent, and then one to three times more at approximately 10-14 day intervals. Disease is rated by observing the ear leaf and the leaf above, then rating the symptoms on a 1 to 9 scale where the maximum score of nine indicates no disease symptoms and the minimum score of one indicates complete death of the plant (Figure 7, reproduced from Kump et al. (2011)). Generally, several plants in each plot are inspected briefly and one representative score is recorded for each plot unless disease severity obviously differs from plant to plant. In general an experienced scorer can rate a plot in about 15 to 20 sec.
Data analysis
The analytical approach will depend on experimental design. Typically, when three or more sets of disease ratings have been recorded over the season, the disease rating for each plot is calculated as an area under disease progress curve (AUDPC) or a standardized AUDPC (sAUDPC). sAUDPC is calculated by taking the average value of two consecutive ratings and multiplying by the number of days between the ratings. Values are then summed over all the intervals and then adjusted by dividing by the number of days of evaluation, so that the sAUDPC scores are on a similar one to nine scale as the initial ratings (Campbell and Madden, 1990). In other cases, the scores from the different scoring dates can be analyzed separately to identify resistance that is apparent at different times of the season (e.g., Balint-Kurti et al., 2006).
Notes
- Northern Leaf Blight inoculation and rating. Exserohilum turcicum (causal agent of Northern Leaf Blight) grows more slowly than C. heterostrophus, so its inoculum can be produced by following the same protocol but allowing two to four extra days for the fungus to grow at each stage (on the PDA plates and on the sterile sorghum). We have found that scoring NLB infection based on a percentage necrotic leaf area over the whole plant is more effective than using a 1 to 9 scale as described for SLB above.
- Gray Leaf Spot inoculation and rating. Cercospora zeae-maydis (causal agent of Gray Leaf Spot) grows more slowly than C. heterostrophus and produces a leathery, dense fungal mat when grown on PDA, which is not easily distributed in the sterile sorghum. To produce inoculum of C. zeae-maydis, following the initial growth on PDA, prepare a suspension by shaking several slices of the fungal culture in a 15-ml sterile tube containing 7.5 ml of 0.05% Tween-20 and several metal balls. Pipet 1 to 2 ml of the suspension onto V8-agar (10% V8 juice, 15 g/L agar, 1g/L CaCO3, autoclave for 30 min) plates, tilt to spread evenly, allow to dry slightly, then Parafilm and incubate for about 2 weeks. Use the V8-agar cultures to inoculate the sterile sorghum. GLS is scored in an essentially identical way to SLB.
Recipes
- V8-agar medium
10% V8 juice
15 g/L agar
1g/L CaCO3
Autoclave at 121 °C for 30 min
Acknowledgments
We would like to thank all our jolly bands of inoculators over the years for sacrificing their backs for the sake of science, especially Greg Marshall. We thank Cathy Herring and her staff at Central Crops Research Station in Clayton NC, where most of these inoculations and ratings were performed. Dr. Martin Carson provided critical guidance and advice in setting up this protocol. We appreciate Brent McCraven’s help with video editing. Luis Lopez-Zuniga took some of the pictures shown in the figures. This work has been funded by USDA-ARS and by NSF grant # 1127076. The authors declare no conflicts of interest or competing interests.
References
- Balint-Kurti, P. J., Krakowsky, M. D., Jines, M. P., Robertson, L. A., Molnar, T. L., Goodman, M. M. and Holland, J. B. (2006). Identification of quantitative trait Loci for resistance to southern leaf blight and days to anthesis in a maize recombinant inbred line population. Phytopathology 96(10): 1067-1071.
- Balint-Kurti, P. J., Wisser, R. and Zwonitzer, J. C. (2008a). Use of an advanced intercross line population for precise mapping of quantitative trait loci for gray leaf spot resistance in maize. Crop Sci 48: 1696-1704.
- Balint-Kurti, P. J., Yang, J., Van Esbroeck, G., Jung, J. and Smith, M. E. (2010). Use of a maize advanced intercross line for mapping of QTL for northern leaf blight resistance and multiple disease resistance. Crop Sci 50: 458-466.
- Balint-Kurti, P. J., Zwonitzer, J. C., Pe, M. E., Pea, G., Lee, M. and Cardinal, A. J. (2008b). Identification of quantitative trait Loci for resistance to southern leaf blight and days to anthesis in two maize recombinant inbred line populations. Phytopathology 98(3): 315-320.
- Balint-Kurti, P. J., Zwonitzer, J. C., Wisser, R. J., Carson, M. L., Oropeza-Rosas, M. A., Holland, J. B. and Szalma, S. J. (2007). Precise mapping of quantitative trait loci for resistance to southern leaf blight, caused by Cochliobolus heterostrophus race O, and flowering time using advanced intercross maize lines. Genetics 176(1): 645-657.
- Belcher, A. R., Zwonitzer, J. C., Santa Cruz, J., Krakowsky, M. D., Chung, C. L., Nelson, R., Arellano, C. and Balint-Kurti, P. J. (2012). Analysis of quantitative disease resistance to southern leaf blight and of multiple disease resistance in maize, using near-isogenic lines. Theor Appl Genet 124(3): 433-445.
- Byrnes, K.J. and Pataky, J. K. (1989). Relationships between yield of three maize hybrids and severity of southern leaf blight caused by race O of Bipolaris maydis. Plant Disease 73: 834-840.
- Campbell, C. L. and Madden, L. V. (1990). Introduction to plant disease epidemiology. John Wiley and Sons pp: P192-194.
- Carson, M. L., Stuber, C. W. and Senior, M. L. (2004). Identification and mapping of quantitative trait Loci conditioning resistance to southern leaf blight of maize caused by Cochliobolus heterostrophus race O. Phytopathology 94(8): 862-867.
- Chung, C. L., Longfellow, J. M., Walsh, E. K., Kerdieh, Z., Van Esbroeck, G., Balint-Kurti, P. and Nelson, R. J. (2010). Resistance loci affecting distinct stages of fungal pathogenesis: use of introgression lines for QTL mapping and characterization in the maize--Setosphaeria turcica pathosystem. BMC Plant Biol 10: 103.
- Fisher, D. E., Hooker, A. L., Lim, S. M. and Smith, D. R. (1976). Leaf infection and yield loss caused by four Helminthosporium leaf diseases of corn. Phytopathology 66: 942-944.
- Holley, R. N. and Goodman, M. M. (1989). New sources of resistance to southern corn leaf blight from tropical hybrid maize derivatives. Plant Dis 73: 562-564.
- Kump, K. L., Bradbury, P. J., Wisser, R. J., Buckler, E. S., Belcher, A. R., Oropeza-Rosas, M. A., Zwonitzer, J. C., Kresovich, S., McMullen, M. D., Ware, D., Balint-Kurti, P. J. and Holland, J. B. (2011). Genome-wide association study of quantitative resistance to southern leaf blight in the maize nested association mapping population. Nat Genet 43(2): 163-168.
- Mueller, D. S., Wise, K. A., Sisson, A. J., Allen, T. W., Bergstrom, G. C., Bosley, B., Bradley, C. A., Byamukama, E. C., Chilvers, M. I., Collins, A., Faske, T., Friskop, A. J., Hollier, C. A., Isakeit, T., Jackson-Ziems, T. A., Jardine, D. J., Kinzer, K., Koenning, S. R., Malvick, D. K., Meyer, R. F., McMullen, M., Mostrom, M. S., Paul, P., Robertson, A. E., Roth, G. W., Smith, D. L., Tande, C. A., Tenuta, A., Vincelli, P. and Warner, F. (2016). Corn disease loss estimates from the United States and Ontario, Canada from 2012 to 2015. Plant Health Progress.
- Negeri, A. T., Coles, N. D., Holland, J. B. and Balint-Kurti, P. J. (2011). Mapping QTL controlling southern leaf blight resistance by joint analysis of three related recombinant inbred line populations. Crop Sci 51: 1571-1579.
- Poland, J. A. and Nelson, R. J. (2011). In the eye of the beholder: the effect of rater variability and different rating scales on QTL mapping. Phytopathology 101(2): 290-298.
- Rife, T. W. and Poland, J. A. (2014). Field book: an open-source application for field data collection on android. Crop Sci 54: 1624-1627.
- Santa-Cruz, J. H., Kump, K. L., Arellano, C., Goodman, M. M., Krakowsky, M. D., Holland, J. B. and Balint-Kurti, P. J. (2014). Yield effects of two southern leaf blight resistance Loci in maize hybrids. Crop Sci 54: 882-894.
- Ullstrup, A. J. (1972). The impacts of the southern corn leaf blight epidemics of 1970-1971. Annu Revi Phytopathol 10: 37-50.
- Wang, M., Wang, S., Ma, J., Yu, C., Gao, J. and Chen, J. (2017). Detection of Cochliobolus heterostrophus races in South China. J Phytopathology 165: 681-691.
- Yang, Q., He, Y., Kabahuma, M., Chaya, T., Kelly, A., Borrego, E., Bian, Y., El Kasmi, F., Yang, L., Teixeira, P., Kolkman, J., Nelson, R., Kolomiets, M., J, L. D., Wisser, R., Caplan, J., Li, X., Lauter, N. and Balint-Kurti, P. (2017). A gene encoding maize caffeoyl-CoA O-methyltransferase confers quantitative resistance to multiple pathogens. Nat Genet 49(9): 1364-1372.
- Zwonitzer, J. C., Bubeck, D. M., Bhattramakki, D., Goodman, M. M., Arellano, C. and Balint-Kurti, P. J. (2009). Use of selection with recurrent backcrossing and QTL mapping to identify loci contributing to southern leaf blight resistance in a highly resistant maize line. Theor Appl Genet 118(5): 911-925.
- Zwonitzer, J. C., Coles, N. D., Krakowsky, M. D., Arellano, C., Holland, J. B., McMullen, M. D., Pratt, R. C. and Balint-Kurti, P. J. (2010). Mapping resistance quantitative trait Loci for three foliar diseases in a maize recombinant inbred line population-evidence for multiple disease resistance? Phytopathology 100(1): 72-79.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sermons, S. M. and Balint-Kurti, P. J. (2018). Large Scale Field Inoculation and Scoring of Maize Southern Leaf Blight and Other Maize Foliar Fungal Diseases. Bio-protocol 8(5): e2745. DOI: 10.21769/BioProtoc.2745.
Category
Plant Science > Plant immunity > Disease bioassay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link












