- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Analysis of Direct Interaction between Viral DNA-binding Proteins by Protein Pull-down Co-immunoprecipitation Assay
Published: Vol 8, Iss 1, Jan 5, 2018 DOI: 10.21769/BioProtoc.2678 Views: 12443
Reviewed by: Dennis NürnbergSeda EkiciAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Construction of a Highly Diverse mRNA Library for in vitro Selection of Monobodies
Taishi Kondo [...] Hiroshi Murakami
Aug 20, 2021 3859 Views

Isolation of Antigen-Specific Nanobodies From Synthetic Libraries Using a Protein Selection Strategy That Combines MACS-Based Screening of YSD and FLI-TRAP
Apisitt Thaiprayoon [...] Dujduan Waraho-Zhmayev
Jan 20, 2026 443 Views
Abstract
This protocol analyzes the direct interaction between two DNA-binding proteins by pull-down co-immunoprecipitation. One of the proteins is overexpressed in E. coli as HA-tagged recombinant protein and cell-free extracts are immunoprecipitated in HA-affinity resin. Cell extracts are treated with nuclease to degrade DNA and RNA, which rules out nucleic acid-mediated indirect interaction. Then, a second immunoprecipitation step is performed using the purified putative partner protein. Co-immunoprecipitated proteins can be detected either by Coomassie Blue staining and/or Western blotting (WB) if a specific antibody is available. Moreover, many DNA/RNA binding proteins are highly electropositive, which can hinder WB under standard conditions, as has been shown in histones and histone-like proteins. In this case, we show that the high isoelectric point of the putative partner results in a poor transfer. Tips to troubleshot WB transfer of highly electropositive DNA-binding proteins are provided.
Keywords: Co-immunoprecipitationBackground
Co-immunoprecipitation is a commonly used method to analyze protein-protein interactions (PPIs). Many co-immunoprecipitation protocols use bacteria-expressed proteins. However, the use of cell extracts does not preclude indirect interactions mediated by third proteins or, in the case of DNA/RNA binding proteins, nucleic acids.
Terminal protein of tectivirus Bam35 (B35TP) contains the conserved Tyrosine 194 that provides the OH group to anchor the first 5’-dTMP of the viral genome during protein-primed DNA replication. Moreover, B35TP has strong DNA-binding capacity and, like many DNA-binding proteins, it has a very high isoelectric point (about 10.6), which affect its stability and function in vitro (Berjón-Otero et al., 2016).
The aim of this protocol was to confirm the direct interaction between B35TP and the viral protein P1, a putative transcription factor, originally detected in a genome-wide yeast two hybrid screening (Berjón-Otero et al., 2017). We avoided indirect interactions mediated by nucleic acids by using benzonase-treated cell extracts to immunoprecipitate the bait protein (P1) and the purified recombinant protein as the interacting partner (B35TP).
Materials and Reagents
- Bacterial cell-free extract preparation
- 1.5 ml microcentrifuge tubes (SARSTEDT, catalog number: 72.690 )
- E. coli BL21(DE3) (New England Biolabs, catalog number: C2527I )
- Glycerol (Sigma-Aldrich, catalog number: G5516 )
- LB Broth (Sigma-Aldrich, catalog number: L3522 )
- Ampicillin (Sigma-Aldrich, catalog number: A9518 )
- Glucose (Sigma-Aldrich, catalog number: G5516 )
- TYM-5052 autoinduction medium (Studier, 2005) (ForMedium, catalog number: AIMLB0205 )
- cOmpleteTM ULTRA Tablets, EDTA-free, glass vials Protease Inhibitor Cocktail (Sigma-Aldrich, Roche Diagnostics, catalog number: 05892953001 )
- Lysozyme from chicken egg white (Sigma-Aldrich, catalog number: L6876 )
- Benzonase® Nuclease (Sigma-Aldrich, catalog number: E1014 )
- Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670 )
- Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E6758 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S7907 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: 1551139 )
- Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 435570 )
Note: This product has been discontinued. - Phosphate buffered saline pH 8 (PBS pH 8) (see Recipes)
- 1.5 ml microcentrifuge tubes (SARSTEDT, catalog number: 72.690 )
- Co-immunoprecipitation
- 1.5 ml microcentrifuge tubes (SARSTEDT, catalog number: 72.690 )
- Pierce® Anti-HA agarose (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 26181 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A4503 )
- Glycine (Sigma-Aldrich, catalog number: G8898 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333 )
- Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S7907 )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: 1551139 )
- Tween® 20 (Sigma-Aldrich, catalog number: P1379 )
- Sodium dodecyl sulfate (SDS) (AppliChem, catalog number: A2572 )
- Trizma base (Sigma-Aldrich, catalog number: T1503 )
- 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- Glycerol (Sigma-Aldrich, catalog number: G5516 )
- Bromophenol blue (Sigma-Aldrich, catalog number: B0126 )
- Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 435570 )
- Phosphate buffered saline pH 8 (PBS pH 8) (see Recipes)
- Phosphate buffered saline pH 8 with Tween (PBS-T pH 8) (see Recipes)
- 4x Laemmli SDS-PAGE sample buffer (see Recipes)
- 1.5 ml microcentrifuge tubes (SARSTEDT, catalog number: 72.690 )
- Western-blot
- Immobilon-P Membrane, PVDF, 0.45 µm, 26.5 x 3.75 m roll (Merck, catalog number: IPVH00010 )
- Grade 3MM Chr Blotting Paper, sheet, 46 x 57 cm (GE Healthcare, catalog number: 3030-917 )
- X-ray film (VWR, catalog number: 11299-022 )
Manufacturer: Associated Metals, catalog number: UPM0810 . - SeeBlueTM Plus2 Pre-stained Protein Standard (Thermo Fischer Scientific, InvitrogenTM, catalog number: LC5925 )
- Coomassie Blue R250 (Sigma-Aldrich, catalog number: 27816 )
- Methanol (Sigma-Aldrich, catalog number: 32213 )
- Antibodies (e.g., anti-TP serum raised in rabbits, goat anti-rabbit horseradish peroxidase-conjugate antibody [GE Healthcare, catalog number: RPN4301 ])
- ECLTM Blotting Reagents (GE Healthcare, catalog number: RPN2109 )
- Trizma® base (Sigma-Aldrich, catalog number: T1503 )
- Glycine for molecular biology (AppliChem, catalog number: A1067 )
- Sodium dodecyl sulfate (SDS) (AppliChem, catalog number: A2572 )
- Low fat milk powder (e.g., Nestle Sveltesse)
- Acrylamide/Bis Solution, 37.5:1 (40% w/v), 2.6% C (SERVA Electrophoresis, catalog number: 10681.01 )
- Ammonium peroxodisulfate for analysis EMSURE® ACS, Reag. Ph Eur. (APS) (Merck, catalog number: 1012010500 )
- N,N,N’,N’-Tetramethyl ethylenediamine (TEMED) GR for analysis (Merck Millipore, catalog number: 1107320100 )
- Phosphate buffered saline pH 7.5 (PBS) (see Recipes)
- Phosphate buffered saline pH 7.5 with Tween (PBS-T) (see Recipes)
- 4x Laemmli SDS-PAGE sample buffer (see Recipes)
- Western electrophoresis buffer (see Recipes)
- Blocking solution (see Recipes)
- SDS-PAGE running buffer (see Recipes)
- Polyacrylamide gel with 5% stacking gel and 15% running gel (see Recipes)
- Transfer buffers (see Recipes)
- Western transfer buffer
- Standard transfer buffer
- Modified transfer buffer
- Western transfer buffer
- Immobilon-P Membrane, PVDF, 0.45 µm, 26.5 x 3.75 m roll (Merck, catalog number: IPVH00010 )
Equipment
- 50 ml flasks (v.g. VWR, catalog number: 214-1130 )
- Micropipettes (Gilson, model: PIPETMAN, catalog numbers: F144801 , F144600 , F144601 and F144802 )
- Incubator shaker for bacterial cultures
- Refrigerated centrifuge (Hettich, model: MIKRO 22R )
- Thermomixer compact (Eppendorf, catalog number 5386000010 )
- Gel electrophoresis chamber (Mini-Protean®, Bio-Rad Laboratories, catalog number: 1658004 )
- Tank blot device (Mini Trans-Blot® Electrophoretic Transfer cell, Bio-Rad Laboratories, catalog number: 1703930 )
- Rotating wheel
- Developer (Kodak, model: X-OMAT 2000 processor ) or WB documentation system
- Autoradiography Cassette (Amersham Hypercassette)
- Sonicator (Sartorius, model: LABSONIC® M )
Procedure
This protocol analyzes the direct interaction between two DNA-binding proteins by pull-down co-immunoprecipitation (Figure 1). In this protocol one of the proteins, P1 from bacteriophage Bam35, is overexpressed in E. coli as an HA-tagged recombinant protein and bound to the HA-affinity resin. Three different IPs are performed, using Nt- or Ct-tagged P1 with the HA motif (HA-P1 and P1-HA, respectively), as well as the empty plasmid as a negative control.
After a washing step, the putative partner protein, B35TP, is incubated with the P1 bound to the resin. This might also be done using both extracts or both purified proteins. We advise not to use cell extracts in the second step in order to avoid false positives derived from interactions mediated by third proteins with the HA-tagged recombinant protein.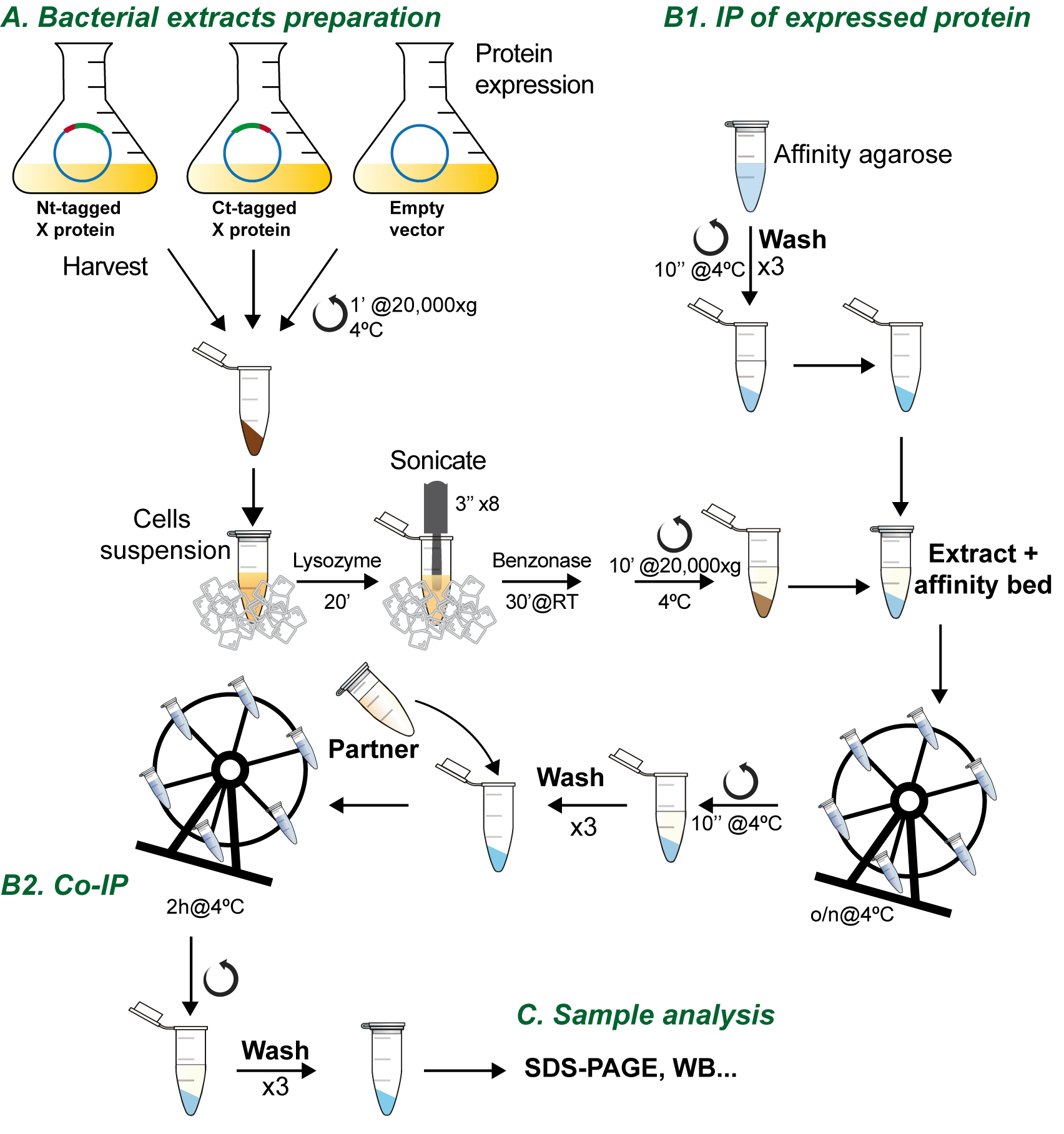
Figure 1. Flow chart of the co-immunoprecipitation procedure
- Bacterial cell-free extract preparation (Figure 1A)
- Inoculate a starter culture of E. coli BL21(DE3) harboring the corresponding expression vector or the empty plasmid (as a control to reduce false positives) from a -80 °C glycerol stock in 10 ml LB with 150 µg/ml of ampicillin and 40 mM glucose in 50 ml flasks. Grow overnight at 37 °C, 200 rpm. Different expression vectors may require different antibiotics.
- Dilute saturated cultures 1:100 in 10 ml of fresh TYM-5052 media supplemented with 150 µg/ml of ampicillin and incubate at 30 °C for 16 h in 50 ml flasks. This step may require optimization for each particular recombinant protein.
- Harvest the bacterial cells by centrifugation for 1 min at 20,000 x g at 4 °C. If required pelleted cells could be stored at -80 °C.
- Resuspend pellet in 450 µl of sterile PBS pH 8 (see Recipes) supplemented with protease inhibitor.
Notes:- PBS at pH 8 was used because of the high isoelectric point of many DNA-binding proteins, which in the case of B35TP strongly affects its stability and function in vitro (Berjón-Otero et al., 2016). This may need to be optimized for each protein.
- Protease inhibitor tablets can be added directly to the buffer according to the manufacturer’s instructions. However, since we prefer to prepare a small volume of each buffer, we prepared a 5x stock in PBS buffer (kept at -80 °C) and diluted up to 1x prior to use.
- PBS at pH 8 was used because of the high isoelectric point of many DNA-binding proteins, which in the case of B35TP strongly affects its stability and function in vitro (Berjón-Otero et al., 2016). This may need to be optimized for each protein.
- Add 50 µl of lysozyme 10 mg/ml and incubate for 20 min on ice.
- Disrupt the cells by sonication.
- Set the sonicator at amplitude of 18 µ.
- Sonicate the bacterial suspension on ice for 2-5 sec.
- Keep on ice for 15-30 sec to avoid overheating of the sample.
- Repeat 6-8 times Steps A6b and A6c.
- Set the sonicator at amplitude of 18 µ.
- Add 1.5 µl of benzonase and 2.5 µl of 500 mM MgCl2 and incubate for 30 min at room temperature. Stop the reaction by adding 1.5 µl of 500 mM EDTA.
Note: This step is essential to rule out DNA-mediated indirect interactions between DNA-binding proteins. - Centrifuge for 10 min at 20,000 x g at 4 °C to pellet cellular debris.
- Transfer the supernatant containing the cell-free protein extract to a new 1.5 ml microcentrifuge tube. We recommend performing the immunoprecipitation on the same day.
- Inoculate a starter culture of E. coli BL21(DE3) harboring the corresponding expression vector or the empty plasmid (as a control to reduce false positives) from a -80 °C glycerol stock in 10 ml LB with 150 µg/ml of ampicillin and 40 mM glucose in 50 ml flasks. Grow overnight at 37 °C, 200 rpm. Different expression vectors may require different antibiotics.
- Co-immunoprecipitation
- Immunoprecipitation of the bait protein expressed in bacteria
Prepare the required tubes by adding 50 µl of Anti-HA agarose to a 1.5 ml microcentrifuge tube. In this case, we used three tubes for the extracts of bacteria expressing HA-P1 and P1-HA, and the negative control.- Centrifuge for 10-20 sec at 12,000 x g at 4 °C and discard the supernatant.
- Wash the resin twice with one resin volume of PBS pH 8 supplemented with complete protease inhibitor.
- Centrifuge for 10-20 sec at 12,000 x g at 4 °C and discard the supernatant.
- Add the bacterial cell-free extract from Procedure A to the resin. Incubate the mixture at 4 °C for 18 h on a rotating wheel.
- Spin briefly (10-20 sec, 12,000 x g) at 4 °C and keep the supernatant for analysis of binding efficiency. Samples for analysis can be stored either on ice or at -20 °C for a longer period of time.
- Wash the resin with ten volumes of PBS-T pH 8 (see Recipes) for 5 min at 4 °C on a rotating wheel. Spin briefly (10-20 sec, 12,000 x g) at 4 °C and repeat three times. Keep the supernatant of the three washing steps for analysis.
- Centrifuge for 10-20 sec at 12,000 x g at 4 °C and discard the supernatant.
- Pull-down co-immunoprecipitation of putative interacting proteins
- Add a mixture of 2 µg of the purified putative partner protein and 30 µg of bovine serum albumin in 500 µl of PBS-T to the resin and incubate at 4 °C for 2 h on a rotating Wheel. We used purified B35TP as partner protein, which was purified and quantified as described in Berjón-Otero et al., 2016.
- Wash the resin as before (Steps B1f-B1g).
Note: It is recommended to transfer the resin to a new tube before the last wash step to discard proteins that may be retained on the plastic. - Elute the bound proteins with 50 µl of 1x Laemmli SDS-PAGE sample buffer (see Recipes).
Note: According to the manufacturer’s instructions, immunoprecipitated proteins can usually be eluted with 0.1 M glycine pH 2.0-2.8 for downstream procedures.
- Add a mixture of 2 µg of the purified putative partner protein and 30 µg of bovine serum albumin in 500 µl of PBS-T to the resin and incubate at 4 °C for 2 h on a rotating Wheel. We used purified B35TP as partner protein, which was purified and quantified as described in Berjón-Otero et al., 2016.
- Immunoprecipitation of the bait protein expressed in bacteria
- Western blotting
- Add 4 µl of 4x Laemmli SDS-PAGE sample buffer to 10 µl of binding and washing samples.
- Heat all samples (elution samples included) to 95-100 °C for 3 min and spin briefly (10 sec at 10,000 x g).
- Load samples onto an SDS-PAGE gel (5 µl of the elution sample and the entire volume of binding and washing samples) and load 6 μl eBlueTM Plus2 Pre-stained Protein Standard to determine molecular weights.
- Electrophoresis at constant 150 V for one hour.
Note: Gels can be stained by incubation for 1 h in Coomassie Blue solution and destained by incubation for 1 h in destaining solution instead of/besides doing Western blotting. For staining protocol see He (2011). - Transfer to PVDF membrane using transfer buffer (see Recipes) and pieces of Grade 3MM Chr Blotting Paper (constant 100 V for 90 min at 4 °C).
Notes:- In this case, because of the high isoelectric point of the protein, a modified transfer buffer with 0.025% SDS and without methanol was used (see Recipes and Figure 2).
- To improve the transfer result, it is highly recommended to equilibrate both gel and membrane in the corresponding transfer buffer (5 min at room temperature).
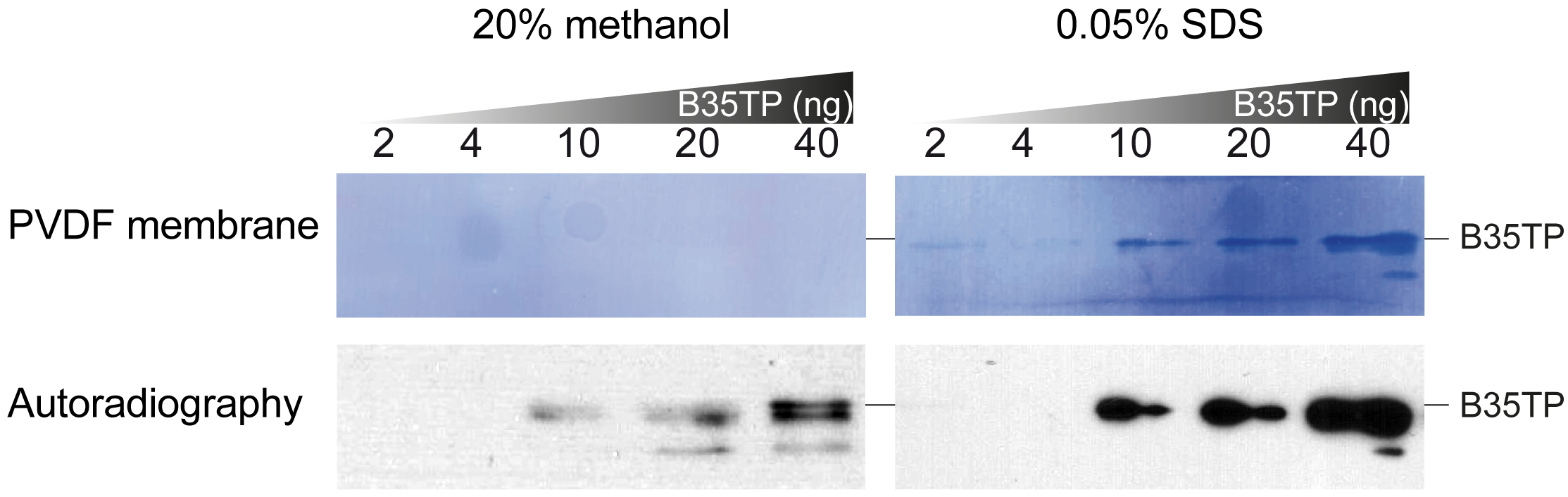
Figure 2. Effect of WB transfer buffer on B35TP detection. Increasing amounts of B35TP were separated by 15% SDS-PAGE and wet-transferred for 40 min either in standard transfer buffer (see Recipes) or in modified transfer buffer (see Recipes). The range of detectable protein amount may need to be optimized. WB was carried out as described in the text and, after detection (lower panels), PVDF membranes were stained with Coomassie Blue solution (upper panels).
- In this case, because of the high isoelectric point of the protein, a modified transfer buffer with 0.025% SDS and without methanol was used (see Recipes and Figure 2).
- Wash membrane with PBS for 20 sec.
- Incubate membrane in 100% methanol for 20 sec and dry at room temperature.
- Incubate membrane in 20 ml of blocking solution (see Recipes) for 30 min at room temperature or overnight at 4 °C.
- Incubate membrane with the primary antibody (concentration depends on the antibody) in 10 ml antibody dilution buffer with gentle agitation for 1 h at room temperature.
- Wash three times for 10 min each with 20 ml of PBS-T.
- Incubate membrane with the secondary antibody (concentration depends on the antibody) in 10 ml antibody dilution buffer with gentle agitation for 1 h at room temperature.
- Wash five times for 5 min each with 20 ml of PBS-T.
- Incubate membrane with 2 ml ECLTM Blotting Reagents for 1 min at RT.
- Drain membrane of excess developing solution, wrap in plastic wrap and expose to X-ray film.
- Add 4 µl of 4x Laemmli SDS-PAGE sample buffer to 10 µl of binding and washing samples.
Data analysis
Samples from each step should be analyzed by either SDS-PAGE followed by Coomassie staining and/or WB.
Recipes
Note: Except otherwise indicated, all the solutions can be stored at room temperature for a few months.
- Phosphate buffered saline, pH 7.5/8 (PBS pH 7.5/8)
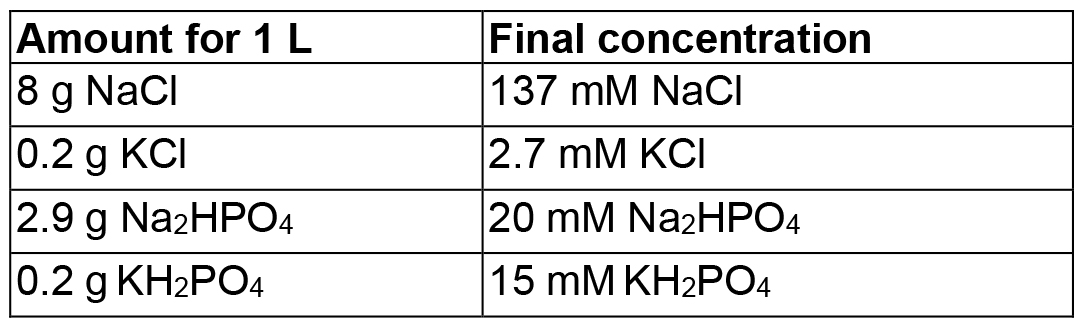
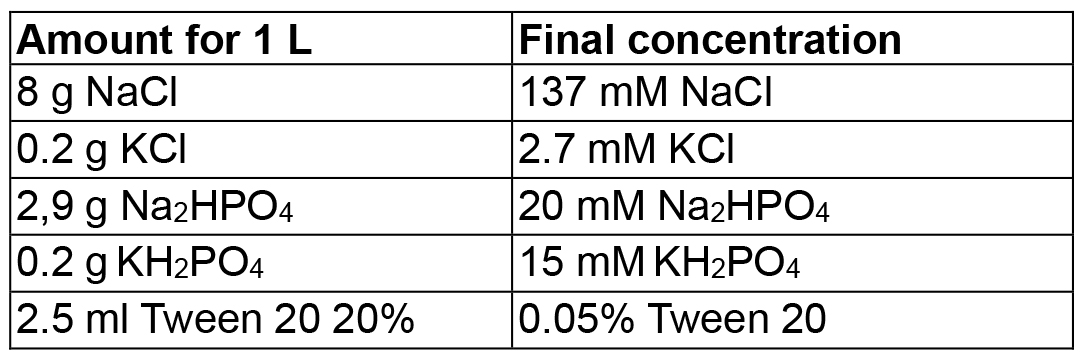
Mix in 800 ml dH2O, adjust pH to 7.5 (or pH 8) with HCl, then adjust volume to 1 L - Phosphate buffered saline pH 7.5/8 with Tween (PBS-T pH 7.5/8)
Mix in 800 ml dH2O, adjust pH to 7.5 (or pH 8) with HCl, then adjust volume to 1 L - 4x Laemmli SDS-PAGE sample buffer
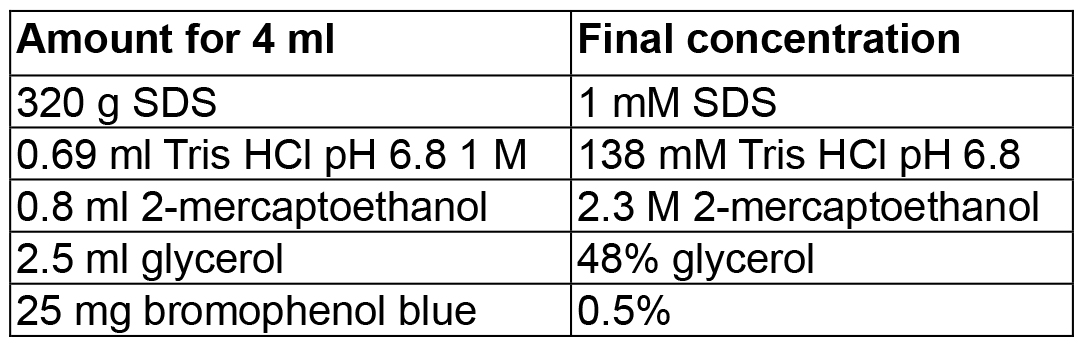
Mix in 4 ml dH2O, then adjust volume to 5 ml - Western blot electrophoresis buffer
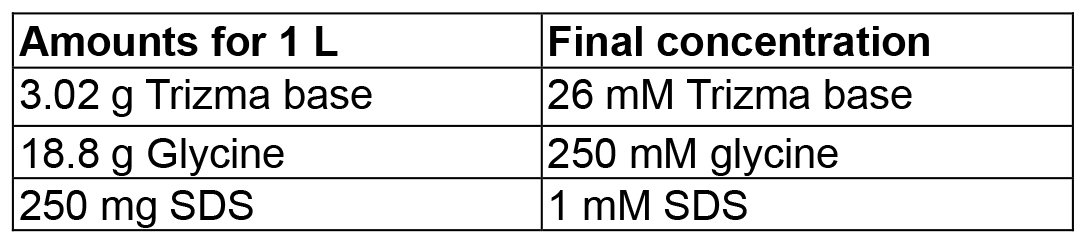
- Blocking solution (use fresh-made)
1 g low fat milk powder
Mix in 80 ml PBS-T, adjust volume to 100 ml - SDS-PAGE running buffer
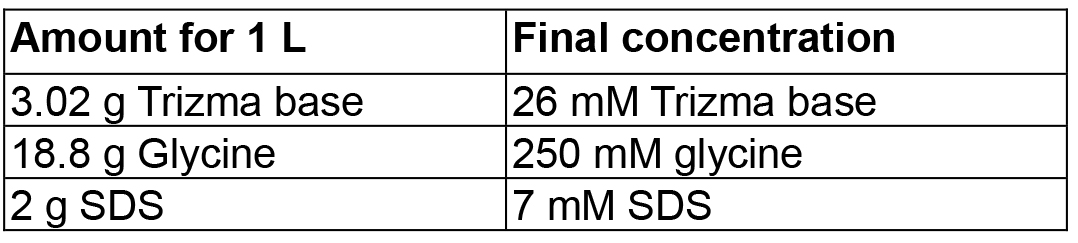
Mix in 800 ml dH2O, adjust volume to 1 L - Polyacrylamide gel with 5% stacking gel and 15% running gel

- Transfer buffers (amount for 1 L)
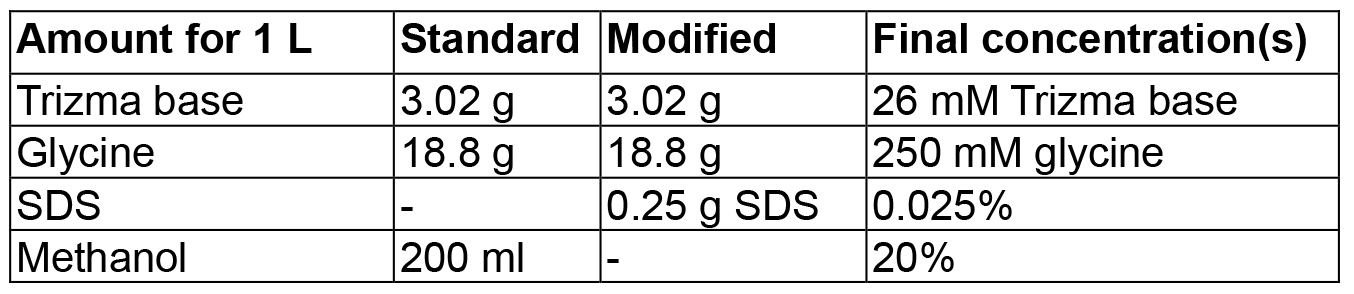
Mix in 800 ml dH2O, adjust volume to 1 L
Acknowledgments
This protocol describes the methodology used in the original paper (Berjón-Otero et al., 2017). This work was supported by Spanish Ministry of Economy and Competitiveness [BFU2014-52656P to M.S.] and ComFuturo Grant from Fundación General CSIC [NewPols4Biotech to M.R-R.]. M.B-O. and A.L. were holders of PhD fellowships FPI [BES-2012-052228] and FPU [15/05797] from the Spanish Economy and Competitiveness and Education Ministries, respectively. An institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa is also acknowledged. The authors do not have any conflict of interest or competing interests to declare
References
- Berjón-Otero, M., Lechuga, A., Mehla, J., Uetz, P., Salas, M. and Redrejo-Rodriguez, M. (2017). Bam35 tectivirus intraviral interaction map unveils new function and localization of phage ORFan proteins. J Virol.
- Berjón-Otero, M., Villar, L., Salas, M. and Redrejo-Rodriguez, M. (2016). Disclosing early steps of protein-primed genome replication of the Gram-positive tectivirus Bam35. Nucleic Acids Res 44(20): 9733-9744.
- He, F. (2011). Coomassie blue staining. Bio Protoc e78.
- Studier, F. W. (2005). Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41(1): 207-234.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lechuga, A., Berjón-Otero, M., Salas, M. and Redrejo-Rodríguez, M. (2018). Analysis of Direct Interaction between Viral DNA-binding Proteins by Protein Pull-down Co-immunoprecipitation Assay. Bio-protocol 8(1): e2678. DOI: 10.21769/BioProtoc.2678.
Category
Microbiology > Microbial biochemistry > Protein > Immunodetection
Biochemistry > Protein > Interaction > Protein-protein interaction
Biochemistry > Protein > Immunodetection > Immunoprecipitation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











