- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Generation of Busulfan Chimeric Mice for the Analysis of T Cell Population Dynamics
(*contributed equally to this work) Published: Vol 7, Iss 24, Dec 20, 2017 DOI: 10.21769/BioProtoc.2650 Views: 10706
Reviewed by: Pooja AroraMartin V KolevAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Ex Vivo Testing of CD8+ T-Cell Division and Activation Using Mouse Splenocytes
Melissa Dolan [...] John M.L. Ebos
Aug 20, 2025 3859 Views

Detection of Autophagy in Human Peripheral Blood Mononuclear Cells Using Guava® Autophagy and Flow Cytometry
Melanie Scherer [...] Jörg Bergemann
Sep 20, 2025 1413 Views

Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
Nov 5, 2025 1442 Views
Abstract
This protocol was developed to generate chimeric mice in which T lymphocytes could be stratified by age on the basis of congenic marker expression. The conditioning drug busulfan is used to ablate host haematopoietic stem cells while leaving the peripheral immune system intact. Busulfan treatment is followed by bone marrow transplantation (BMT), with T-cell depleted donor bone marrow bearing a different congenic marker (CD45.2) to that of the host mouse (CD45.1). New cell production post-BMT can thus be tracked by measuring the fraction of CD45.2+ cells over time within a population of interest (Hogan et al., 2015; Gossel et al., 2017).
Keywords: T cellsBackground
Bone marrow chimeras are a valuable tool for studying immune system development and function. Typically, chimeras are generated by irradiation of host mice followed by transplantation with donor bone marrow. Irradiation causes considerable damage to the haematopoietic system, and full immune reconstitution is delayed by weeks to months post-transplant (Fry and Mackall, 2005). The resulting period of lymphopenia drives spontaneous proliferation of naïve T cells and the acquisition of a memory-like phenotype (Goldrath et al., 2004). To avoid the perturbation of immune homeostasis caused by irradiation, we turned our attention to the conditioning drug busulfan. Busulfan is an alkylating agent that is toxic to haematopoietic stem cells (HSC) but does not deplete circulating lymphocytes (Westerhof et al., 2000; Hsieh et al., 2007). Following busulfan conditioning and bone marrow transplantation (BMT), chimeras are indistinguishable from untreated controls: they have normal numbers of naïve and memory CD4 and CD8 T cells, and these cells express normal levels of the proliferation marker Ki67 (Hogan et al., 2015; Gossel et al., 2017). Therefore, busulfan conditioning allows induction of chimerism while preserving peripheral immune homeostasis. By using donor bone marrow bearing a congenic marker, it is possible to distinguish host- or donor-origin cells by flow cytometry. While HSC replacement is never absolute, emergence of donor origin cells can be used as an accurate proxy for de novo haematopoetic development since BMT, thereby allowing cells within a population to be stratified by age on the basis of congenic marker expression.
Materials and Reagents
- 5 ml syringes (BD, catalog number: 302187 )
- 25 G needles (Terumo, catalog number: GS-351 )
- Insulin syringes (B. Braun Melsungen, catalog number: 9151125 )
- Petri dishes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 150288 )
- 70 μm cell strainers (Corning, Falcon®, catalog number: 352350 )
- 50 ml tubes (Corning, Falcon®, catalog number: 352070 )
- FACS tubes with lid (Corning, Falcon®, catalog number: 352058 )
- Host mice: B6 CD45.1 adult females (THE JACKSON LABORATORY, catalog number: 002014 )
- Donor mice: C57BL/6 CD45.2 adult females (THE JACKSON LABORATORY, catalog number: 000664 )
Note: One donor typically provides sufficient T-cell depleted bone marrow for two recipients.
- Busilvex® 6 mg/ml busulfan concentration for infusion (Pierre Fabre)
- Biotinylated anti-TCRβ (Thermo Fisher Scientific, eBioscienceTM, catalog number: 13-5961 )
- Biotinylated anti-CD3ε (Thermo Fisher Scientific, eBioscienceTM, catalog number: 13-0031 )
- Dynabeads M-280 streptavidin (Thermo Fisher Scientific, InvitrogenTM, catalog number: 11206D )
- Fluorescent streptavidin (for example, Streptavidin APC, BioLegend, catalog number: 405207 )
- Fluorescent antibody to CD45.1 (for example, anti-CD45.1 Brilliant Violet 650, BioLegend, catalog number: 110736 )
- Fluorescent antibody to CD45.2 (for example, anti-CD45.2 PE-Dazzle 594, BioLegend, catalog number: 109846 )
- Phosphate-buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190 )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906 )
- PBS/BSA (see Recipes)
Equipment
- Dissection tools (sharp scissors and forceps)
- Refrigerated benchtop centrifuge
- EasySepTM magnet (STEMCELL Technologies, catalog number: 18000 )
- Tube rotator
- Flow cytometer
Procedure
Note: The steps in this procedure are summarised in Figure 1. All animal procedures were performed in accordance with UK Home Office regulations.

Figure 1. Generation of busulfan chimeric mice. This schematic summarises the principal steps of this protocol for the generation of busulfan chimeric mice. Bone marrow cells from CD45.2+ donor mice are labeled with biotinylated antibodies to CD3 and TCRb, followed by labelling with streptavidin-coated magnetic beads. Labelled cells are immobilised using a magnet, allowing the unbound fraction to be collected. T cell depleted bone marrow cells are then injected into busulfan-conditioned CD45.1+ host mice.
- Busulfan treatment of host CD45.1 mice (Day -2 and Day -1)
- Prepare a fresh working solution of 1 mg/ml busulfan by diluting Busilvex (6 mg/ml busulfan) in sterile PBS.
Note: This must be prepared fresh each day and cannot be stored.
- Ear mark and weigh each mouse individually prior to each injection and calculate the appropriate volume of working solution required to deliver a dose of 10 μg/g busulfan–i.e., 10 μl of 1 mg/ml working solution per gram of body weight.
- Day -2: Deliver appropriate dose of busulfan by intraperitoneal injection.
- Allow host mice 24 h recovery after injection.
- Day -1: Repeat Steps A1-A4 for a second injection of busulfan. The final dose of busulfan in each mouse is thus 20 μg/g, delivered as two doses of 10 μg/g 24 h apart.
Note: Mice remain healthy and alert after busulfan injection. HSC are depleted within 24 h after busulfan treatment (Westerhof et al., 2000).
- Prepare a fresh working solution of 1 mg/ml busulfan by diluting Busilvex (6 mg/ml busulfan) in sterile PBS.
- Preparation of T cell depleted donor CD45.2 bone marrow (Day 0)
Note: Cell suspensions can be prepared on the bench; a laminar flow hood is not required.- Euthanise donor mice by cervical dislocation and remove the femur and tibia from both hind legs.
- Use a 5 ml syringe fitted with a 25 G needle to flush each of the bones with PBS/BSA (see Recipes), collecting the marrow in a Petri dish.
- Filter the bone marrow through a 70 μm cell strainer into a 50 ml tube.
- Centrifuge at 300 x g for 5 min at 4 °C, discard supernatant and resuspend in 1 ml PBS/BSA per donor mouse.
- Count cells (expect to recover ~40 million cells per donor mouse) and adjust the volume of cell suspension to a concentration of 20-40 million cells/ml.
- Add anti-TCRβ and anti-CD3ε biotinylated antibodies to a final concentration of 1 μg/ml for each, incubate with rotation for 30 min at 4 °C.
- Centrifuge at 300 x g for 5 min at 4 °C, discard supernatant and resuspend in PBS/BSA at a concentration of at 20-40 million cells/ml.
- Transfer cell suspension to 5 ml FACS tubes with lids. Use multiple tubes if necessary, with a volume of 3-4 ml per tube.
- Add streptavidin Dynabeads to a bead: cell ratio of approximately 1:1 and incubate with rotation for 30 min at 4 °C.
- Insert the tube of beads with cell suspension into the Easysep magnet and incubate for 2 min on ice.
- Collect the supernatant containing the unbound fraction of cells into a fresh tube.
- Repeat Steps B10-B11 with the collected unbound fraction of cells, to ensure all beads are removed from the cell suspension.
- Centrifuge at 300 x g for 5 min at 4 °C, discard supernatant and resuspend in 1 ml PBS/BSA per donor mouse.
- Count cells, expecting a loss of 10-40% from the original cell count (Step B5) due to depletion and repeated wash steps.
- Confirm depletion of T cells by staining samples of pre- and post-depletion cells with fluorescent streptavidin and analysing by flow cytometry. Expect to see ~5% of lymphocytes staining positive in the pre-depletion sample, reduced to < 0.5% of lymphocytes in the post-depletion sample.
- Euthanise donor mice by cervical dislocation and remove the femur and tibia from both hind legs.
- Bone marrow transplantation (Day 0)
- Allow host mice 24 h recovery after the second injection of busulfan before transplantation with T-cell depleted donor bone marrow.
- Resuspend T-cell depleted bone marrow in PBS to a concentration of at least 50 million cells/ml.
- Deliver 200 μl (i.e., at least 10 million cells) to host mice by intravenous injection into the tail vein.
- Allow host mice 24 h recovery after the second injection of busulfan before transplantation with T-cell depleted donor bone marrow.
Data analysis
- At the desired timepoint(s) post-BMT, sacrifice chimeras and harvest organs of interest for analysis by flow cytometry. Organs and staining panels vary depending on the cell type of interest, but the inclusion of antibodies to both CD45.1 and CD45.2 is necessary in all staining panels to allow positive identification of cells of host (CD45.1+) or donor (CD45.2+) origin.
- By 6 weeks post-BMT, we typically see that the fraction of cells in the thymus that are donor-derived has stabilised at approximately 90%, and this is maintained up to one year post-BMT. The extent of stem cell replacement and therefore chimerism in progenitor populations varies between mice, reflecting variability in the ablation of host stem cells in the bone marrow. Thus the output of de novo generated T cells from the thymus is chimeric with respect to donor and host. To account for this, we measure the donor: host ratio within the thymus CD4+CD8+DP population of all mice as an accurate proxy for stem cell replacement in the bone marrow. With this estimate of stem cell chimerism, it is possible to estimate what fraction of any haematopoietic compartment has been replaced since the time of BMT, so called the fraction of ‘new’ cells, by the following formula:
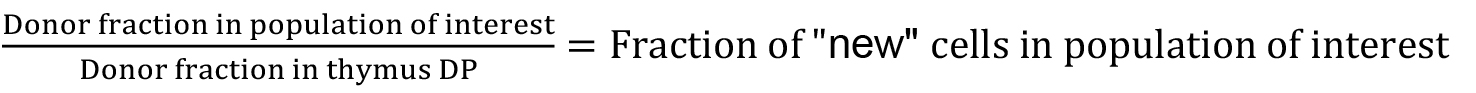
Since this calculation in effect normalises data against variability in stem cell chimerism, such estimates of ‘fraction new’ can be directly compared between individuals regardless of variability of stem cell engraftment following busulfan treatment (Figure 2).
- Timepoints chosen for analysis of chimeras depend upon the cell population of interest. For naïve CD4 and CD8 T cells, older host-derived cells are gradually replaced by new donor-derived cells until the donor:host ratio stabilises at approximately 30 weeks post-BMT (Figure 2D and Hogan et al., 2015). The replacement kinetics of memory CD4 T cell subsets are similar to naïve cells (Gossel et al., 2017), although the level of saturation varies by population, reflecting differences in underlying homeostatic mechanisms.
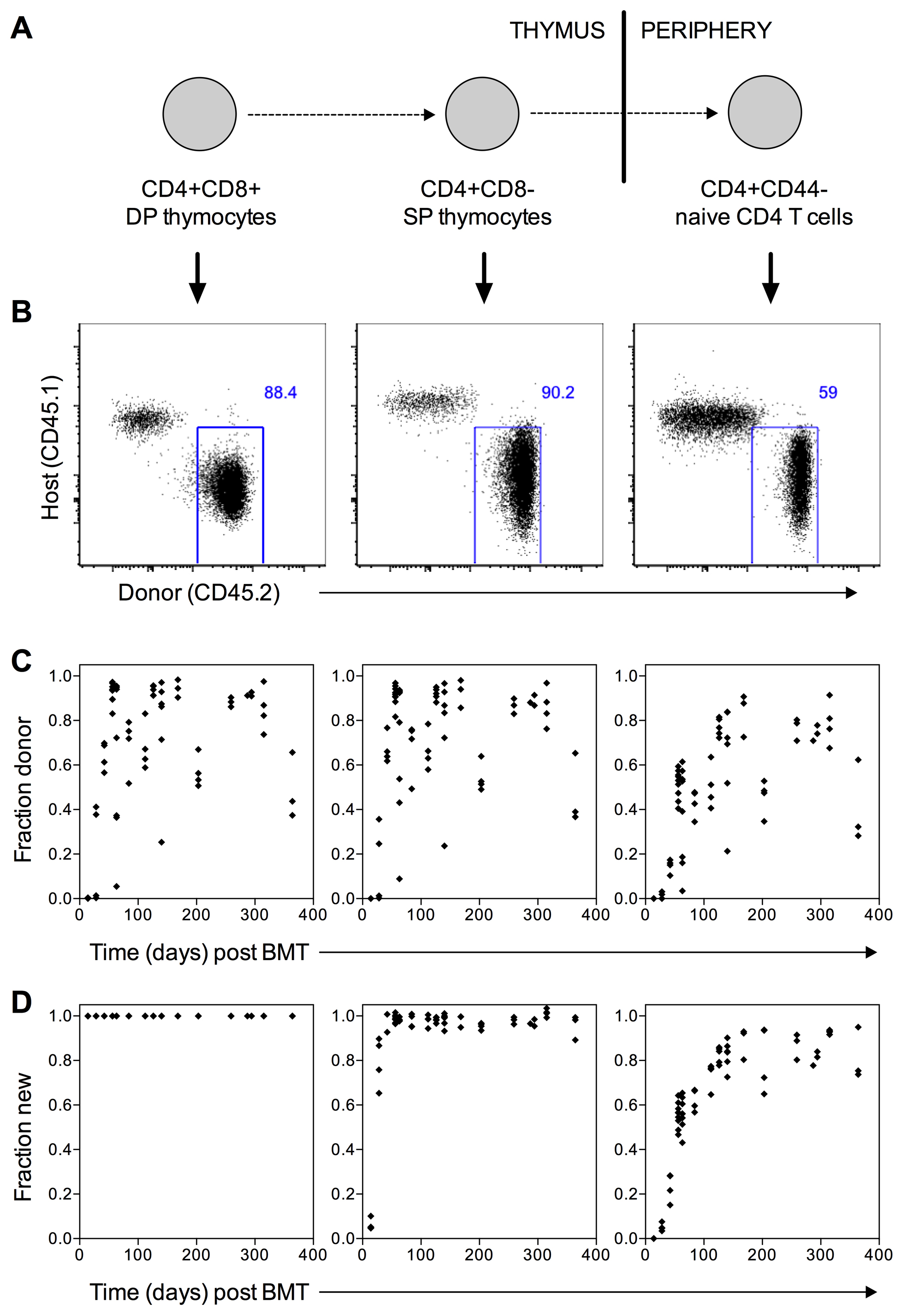
Figure 2. Representative data analysis. A. Schematic of maturation stages for the T cell populations shown in Figures 2B-2D. B. Representative flow cytometry plots showing host (CD45.1+) and donor (CD45.2+) cells in chimeras 8 weeks post-BMT. Left panel shows thymocytes gated on CD4+CD8+ double positive cells; middle panel shows thymocytes gated on CD4+CD8- single positive cells; right panel shows splenocytes gated on naive (CD4+CD44lo) CD4 T cells. B. Fraction of donor cells in DP (left), CD4 SP (middle) and CD4 naive (right) populations at various times post-BMT. Each point represents one mouse. C. Fraction of ‘new’ cells in DP (left), CD4 SP (middle) and CD4 naive (right) populations at various times post-BMT. Each point represents one mouse. ‘Fraction new’ is calculated according to the formula (donor fraction in a population of interest)/(donor fraction in thymus DP).
Notes
- The busulfan dose used in this protocol (20 μg/g) was specifically tested in B6 CD45.1 mice and may require testing and titration in alternative mouse strains.
- Delivery of busulfan as two doses of 10 μg/g with 24 h recovery between injections is typically better tolerated by the mice than a single dose of 20 μg/g.
- The number of bone marrow cells injected can be varied from 10-20 million per mouse. However, doses lower than 10 million cells per mouse may result in a reduced level of chimerism.
Recipes
- PBS/BSA
- Supplement Dulbecco’s PBS with 1% BSA
- Keep sterile and store at 4 °C for up to 1 month
- Supplement Dulbecco’s PBS with 1% BSA
Acknowledgments
This protocol was developed for two published studies (Hogan et al., 2015; Gossel et al., 2017), both supported by the National Institutes of Health (R01 AI093870) and the Medical Research Council (MC-PC-13055). The authors declare no conflicts of interest or competing interests.
References
- Fry, T. J. and Mackall, C. L. (2005). Immune reconstitution following hematopoietic progenitor cell transplantation: challenges for the future. Bone Marrow Transplant 35 Suppl 1: S53-57.
- Goldrath, A. W., Luckey, C. J., Park, R., Benoist, C. and Mathis, D. (2004). The molecular program induced in T cells undergoing homeostatic proliferation. Proc Natl Acad Sci U S A 101(48): 16885-16890.
- Gossel, G., Hogan, T., Cownden, D., Seddon, B. and Yates, A. J. (2017). Memory CD4 T cell subsets are kinetically heterogeneous and replenished from naive T cells at high levels. Elife 6.
- Hogan, T., Gossel, G., Yates, A. J. and Seddon, B. (2015). Temporal fate mapping reveals age-linked heterogeneity in naive T lymphocytes in mice. Proc Natl Acad Sci U S A 112(50): E6917-6926.
- Hsieh, M. M., Langemeijer, S., Wynter, A., Phang, O. A., Kang, E. M. and Tisdale, J. F. (2007). Low-dose parenteral busulfan provides an extended window for the infusion of hematopoietic stem cells in murine hosts. Exp Hematol 35(9): 1415-1420.
- Westerhof, G. R., Ploemacher, R. E., Boudewijn, A., Blokland, I., Dillingh, J. H., McGown, A. T., Hadfield, J. A., Dawson, M. J. and Down, J. D. (2000). Comparison of different busulfan analogues for depletion of hematopoietic stem cells and promotion of donor-type chimerism in murine bone marrow transplant recipients. Cancer Res 60(19): 5470-8.
Article Information
Copyright
Hogan et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Hogan, T., Yates, A. and Seddon, B. (2017). Generation of Busulfan Chimeric Mice for the Analysis of T Cell Population Dynamics. Bio-protocol 7(24): e2650. DOI: 10.21769/BioProtoc.2650.
- Gossel, G., Hogan, T., Cownden, D., Seddon, B. and Yates, A. J. (2017). Memory CD4 T cell subsets are kinetically heterogeneous and replenished from naive T cells at high levels. Elife 6.
Category
Immunology > Animal model > Mouse
Immunology > Immune cell differentiation > T cell
Cell Biology > Cell-based analysis > Flow cytometry
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









