- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Extraction and Molybdenum Blue-based Quantification of Total Phosphate and Polyphosphate in Parachlorella
Published: Vol 7, Iss 17, Sep 5, 2017 DOI: 10.21769/BioProtoc.2539 Views: 11346
Reviewed by: Maria SinetovaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Quick Method to Quantify Iron in Arabidopsis Seedlings
Chandan Kumar Gautam [...] Wolfgang Schmidt
Mar 5, 2022 3922 Views

Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Chuanfeng Ju [...] Zhenqian Zhang
Mar 5, 2023 2040 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1172 Views
Abstract
Inorganic phosphorus is a non-renewable resource and an essential element for life on Earth. Organisms such as algae, protists, and animals can store phosphate (Pi) through uptake of Pi as polyphosphate (poly-P), which is a linear polymer of orthophosphate residues linked by high-energy phosphoanhydride bonds. Here, we describe procedures for extraction of total phosphate and poly-P from Parachlorella cells and quantification of orthophosphate based on molybdenum blue assay. The present method may be applicable for other microalgae.
Keywords: AlgaBackground
Biological phosphorus recovery is a particularly attractive form of nutrient recycling. Algae can accumulate phosphate (Pi), and Pi-enriched algal biomass can be used as biofertilizer (Solovchenko et al., 2016). In a previous study, Ota et al. (2016) revealed the relationship between electron dense bodies and poly-P dynamics under sulfur-deficient (-S) conditions in Parachlorella kessleri. Parachlorella is a genus of green algae in the class Trebouxiophyceae, characterized by a rigid cell wall and an asexual, non-motile life cycle. The protocol presented here allows extraction of total Pi and polyphosphate (poly-P) from Chlorella and quantification of inorganic phosphorus based on molybdenum blue reaction, which is a standard method used to quantify orthophosphate. The theoretical background of the molybdenum blue reaction was reviewed previously by Nagul et al. (2015).
Materials and Reagents
- Pipette tips for 10 µl, 200 µl and 1,000 µl (Labcon, catalog numbers: 1161-965 , 1065-960 , 1168-960 )
- 15-ml conical centrifuge tubes (FUKAEKASEI and WATSON, catalog number: 1332-015S )
- 2-ml microtubes (SARSTEDT, catalog number: 72.695.500 )
- Aluminum foil (Mitsubishi Aluminum, 0.012 mm thick)
- 96-well microplates, non-treated surface (Asahi Glass, catalog number: 1860-096 )
- Microplate seal (qPCR seal) (4titude, catalog number: 4ti-0560 )
- Parachlorella kessleri (National Institute for Environmental Studies, catalog number: NIES-2152 )
- TAP medium (without agar; see http://mcc.nies.go.jp/02medium.html)
- Sodium hypochlorite (available chlorine, min. 5.0%) (Wako Pure Chemical Industries, catalog number: 197-02206 )
- Glass beads, acid-washed 425-600 µm (Sigma-Aldrich, catalog number: G8772 )
- Ethanol (99.5% v/v) (Wako Pure Chemical Industries, catalog number: 057-00456 )
- Potassium peroxodisulfate (K2S2O8) (Kishida Chemical, catalog number: 310-63931 )
- Antimony potassium tartrate trihydrate (C8H4K2O12Sb2·3H2O) (Alfa Aesar, catalog number: A13766 )
- L-Ascorbic acid (Wako Pure Chemical Industries, catalog number: 012-04802 )
- Hexaammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24·4H2O) (Wako Pure Chemical Industries, catalog number: 016-06902 )
- Phosphate ion standard solution (NaH2PO4 in water) (Wako Pure Chemical Industries, catalog number: 168-17461 )
- Sulfuric acid (Wako Pure Chemical Industries, catalog number: 195-04706 )
- Ammonium molybdate tetrahydrate solution (see Recipes)
Equipment
- Micro-spatula (AS ONE, catalog number: 6-524-06 )
- Pipettes for 10 µl, 200 µl and 1,000 µl (Eppendorf, model: Research® plus )
- Microtube mixer (TOMY SEIKO, model: MT-360 )
- Autoclave (TOMY SEIKO, model: LSX-300 )
- Centrifuge, swing rotor (TOMY SEIKO, model: LC-121 )
- Refrigerated microcentrifuge (TOMY SEIKO, model: MX-300 )
- Microplate reader (BioTek Instruments, model: EPOCH )
Procedure
Note: See Figure 1 for an overview from sampling to molybdenum blue reaction.
Figure 1. Overview of the phosphate assay in Chlorella. +S, sulfur-replete medium (control culture); -S, sulfur-depleted medium (experimental culture; for details, see Ota et al., 2016).
- Sampling and preparing algal samples
- Take 10 ml of algal culture [OD595: 1-2 in TAP medium (Ota et al., 2016)] in a 15-ml conical centrifuge tube. Centrifuge for 5 min at 2,500 x g at room temperature with the swing rotor. Discard the supernatant. At this point, additional samples should be taken for dry weight and/or cell number measurement (see Data analysis below).
- Resuspend the pellet with 2 ml distilled water and transfer to a new 2-ml microtube. The suspended samples can be divided into halves: 1 ml for poly-P assay and 1 ml for total-P assay.
- Centrifuge again at room temperature for 5 min at 2,500 x g. Discard the supernatant. At this point, the pellet can be stored in a freezer at -20 °C for further analysis.
- Take 10 ml of algal culture [OD595: 1-2 in TAP medium (Ota et al., 2016)] in a 15-ml conical centrifuge tube. Centrifuge for 5 min at 2,500 x g at room temperature with the swing rotor. Discard the supernatant. At this point, additional samples should be taken for dry weight and/or cell number measurement (see Data analysis below).
- Extraction of poly-P
- Add 1 ml of > 5% sodium hypochlorite to the cell pellet.
- Add 2-3 microspatulas of glass beads (approximately 50 mg) and mix vigorously using a microtube mixer for 10-15 min at 4 °C in a cold room. Centrifuge for 2 min at 14,000 x g at 4 °C. Remove the supernatant.
- Add 1 ml of sodium hypochlorite to the cell pellet. Centrifuge for 2 min at 14,000 x g at 4 °C. Remove the supernatant. Repeat step 3.
Note: The high chain length of polyphosphate is virtually insoluble in alkaline sodium hypochlorite (Sutherland and Wilkinson, 1971). - Add 100 µl of distilled water and incubate for 5 min at room temperature. Centrifuge for 2 min at 14,000 x g at 4 °C. Collect the supernatant (a).
- Add 100 µl of distilled water and incubate for 5 min. Centrifuge for 2 min at 14,000 x g at 4 °C. Collect the supernatant (b).
- Add 1.8 ml of ethanol to the supernatant (a + b) and centrifuge for 10 min at 14,000 x g at 4 °C. In this step, poly-Ps are precipitated as a white pellet at the bottom of the test tube.
- Remove the supernatant carefully, add 500 µl of distilled water, and mix vigorously (poly-P solution).
Note: Amount of distilled water may be adjusted from 50 to 500 µl (OD880 > 0.3; see Data analysis below). - Add 100 µl of 4% (w/v) potassium persulfate to the poly-P solution.
- For hydrolysis to orthophosphate, autoclave the poly-P solution at 121 °C for 20 min without fast exhaust option to avoid loss of samples. Leave the cap open and cover the microtube with aluminum foil when autoclaving.
- The autoclaved samples are now ready to use for molybdenum blue assay (see Procedure D).
- Add 1 ml of > 5% sodium hypochlorite to the cell pellet.
- Extraction of total P
- Resuspend the cell pellet from A3 with 1 ml of distilled water.
- Disrupt samples. Add 2-3 microspatulas of glass beads and mix vigorously using a microtube mixer for 10-15 min at 4 °C in a cold room.
- Add 200 µl of 4% (w/v) potassium persulfate to the sample.
- Autoclave the poly-P solution at 121 °C for 20 min without fast exhaust to avoid loss of samples. Leave the cap open and cover the microtube with aluminum foil when autoclaving.
- The autoclaved samples are now ready to use for molybdenum blue assay (see Procedure D).
Note: The supernatant is used for assay. Normally, there is no need for centrifugation step.
- Resuspend the cell pellet from A3 with 1 ml of distilled water.
- Molybdenum blue reaction in a 96-well microplate
- Pipette 200 µl of diluted sample (196 µl of distilled water + 4 µl of poly-P/total-P samples) per well.
- Add 8 µl of ammonium molybdate tetrahydrate solution (see Recipes below).
- Add 2 µl of 7.2% (w/v) L-ascorbic acid solution.
- Seal with plate seal film and mix well (Invert the plate 3-5 times).
- Incubate for 20 min in the dark at room temperature. The mixture will turn blue in color if orthophosphate is present (Figure 2).
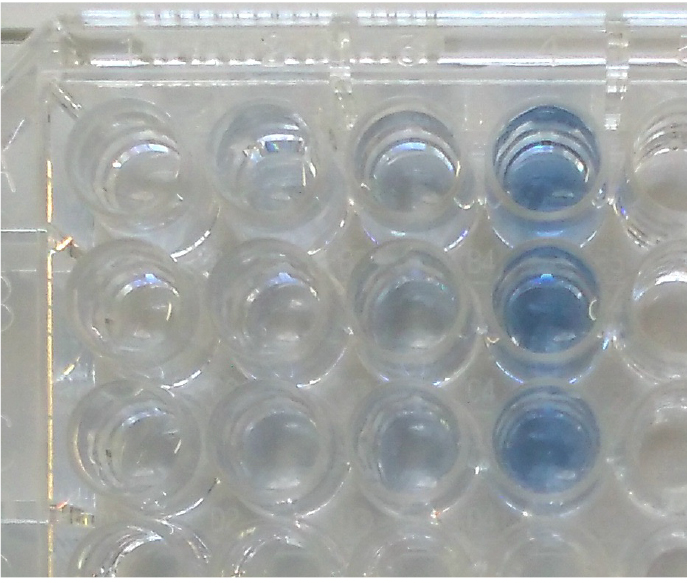
Figure 2. Molybdenum blue reaction in a 96-well microplate. This is an example of the phosphate ion standard dilution series (0, 0.5, 1, 2 mg/L from left to right, n = 3). - Measure the absorbance at 880 nm (OD880) using a microplate reader.
Note: If wavelength at 880 nm is not available, select an alternative wavelength near 880 nm.
- Pipette 200 µl of diluted sample (196 µl of distilled water + 4 µl of poly-P/total-P samples) per well.
Data analysis
A calibration curve is necessary for each experiment for calculation of corresponding absolute values. A phosphate ion standard solution is diluted with distilled water ranging from 0 to 2 mg/L (e.g., 0, 0.1, 0.5, 1, 2 mg/L), where linearity between absorbance (OD880) and Pi concentration is confirmed (Figure 3). Measure OD880 at one point per well, and a mean value is calculated from at least three replicates per sample (n > 3). A calculation example is as follows: The concentration rate in poly-P extraction is 5-fold (Procedure A), 1,000/(100 + 500) - fold (Procedure B), and 4/200 - fold (Procedure D), respectively. Then, the poly-P concentration (mg/L) is calculated as follows: the corresponding value = {(OD880 - 0.05)/0.13} x1/5 x 600/1,000 x 200/4. Dry weight and/or cell number should be measured for normalization when sampling cultures. These values are used for calculating the amount of Pi per dry weight or cell.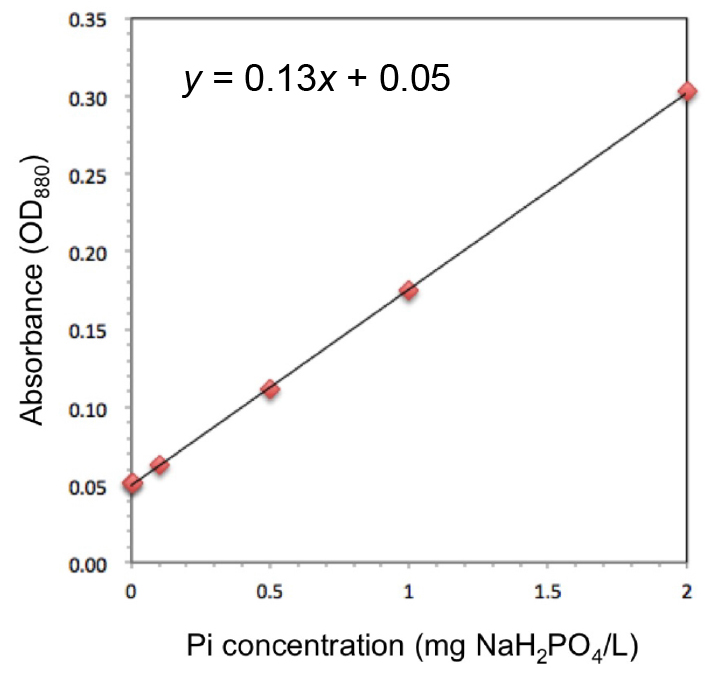
Figure 3. Example of a calibration curve
Notes
- Phosphate ion standard solution can be self-produced using sodium dihydrogenphosphate (NaH2PO4).
- All stock solutions (potassium persulfate, ammonium molybdate tetrahydrate, L-ascorbic acid) should be prepared with distilled water (see also Recipes below).
- Solutions are prepared at time of use. The stock solutions can be stored up to one month at 4 °C.
- Samples should be diluted with distilled water, if OD880 is more than 0.3.
Recipes
- Ammonium molybdate tetrahydrate solution (100 ml)
1.2 g of hexaammonium heptamolybdate tetrahydrate
4.8 mg of potassium antimonyl tartrate sesquihydrate
32 ml of diluted sulfuric acid (sulfuric acid:distilled water = 1:1)
Make up with distilled water to volume
Acknowledgments
This research was supported with funding provided by the CREST program (to SK) of the Japan Science and Technology Agency (JST).
References
- Nagul, E. A., McKelvie, I. D., Worsfold, P. and Kolev, S. D. (2015). The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal Chim Acta 890: 60-82.
- Ota, S., Yoshihara, M., Yamazaki, T., Takeshita, T., Hirata, A., Konomi, M., Oshima, K., Hattori, M., Bisova, K., Zachleder, V. and Kawano, S. (2016). Deciphering the relationship among phosphate dynamics, electron-dense body and lipid accumulation in the green alga Parachlorella kessleri. Sci Rep 6: 25731.
- Solovchenko, A., Verschoor, A. M., Jablonowski, N. D. and Nedbal, L. (2016). Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnol Adv 34(5): 550-564.
- Sutherland, I. W. and Wilkinson, J. F. (1971). Chemical extraction methods of microbial cells. In: Norris, J. R. and Ribbons, D. W. (Eds.). Methods in Microbiology. Vol. 5B. Academic Press pp: 1-665.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ota, S. and Kawano, S. (2017). Extraction and Molybdenum Blue-based Quantification of Total Phosphate and Polyphosphate in Parachlorella. Bio-protocol 7(17): e2539. DOI: 10.21769/BioProtoc.2539.
Category
Plant Science > Plant biochemistry > Other compound
Biochemistry > Other compound > Ion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









