- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Vaginal HSV-2 Infection and Tissue Analysis
Published: Vol 7, Iss 13, Jul 5, 2017 DOI: 10.21769/BioProtoc.2383 Views: 10521
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vivo Electroporation of Skeletal Muscle Fibers in Mice
Steven J. Foltz [...] Hyojung J. Choo
Jul 5, 2023 1823 Views

Cochlear Organ Dissection, Immunostaining, and Confocal Imaging in Mice
Chenyu Chen [...] Dongdong Ren
Jan 20, 2025 3758 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2627 Views
Abstract
The vaginal murine HSV-2 infection model is very useful in studying mucosal immunity against HSV-2 (Overall et al., 1975; Renis et al., 1976; Parr and Parr, 2003). Histologically, the vagina of Depo-Provera-treated mice is lined by a single layer of mucus secreting columnar epithelial cells overlying two to three layers of proliferative cells. Even though this is morphologically different from the human vagina, it closely resembles the endocervical epithelium, which is thought to be the primary site of HSV-2 infection in women (Parr et al., 1994; Kaushic et al., 2011). In the protocol presented here, mice are pre-treated with Depo-Provera before intra-vaginal inoculation with HSV-2. The virus replicates in the mucosal epithelium from where it spreads to and replicates in the CNS including the spinal cord, brain stem, cerebrum and cerebellum. Cytokine responses can be detected in vaginal washings using ELISA or in vaginal tissues using qPCR. Further, the recruitment of leukocytes to the vagina can be determined by flow cytometry. The model is suitable for research of both innate and adaptive immunity to HSV-2 infection.
Keywords: ImmunologyBackground
Vaginal infection with HSV-2 has been studied in various animal models, such as rabbits, hamsters, guinea pigs, mice and monkeys with viral replication at the peripheral site and retrograde transport of virus to the neurons (Nahmias et al., 1971; Overall et al., 1975; Renis, 1977; Stanberry et al., 1982; Roizman et al., 2013). There are several pros and cons regarding the different animal models in terms of susceptibility to infection, latency, spontaneous reactivation of HSV-2 and availability of animals, especially with regard to the accessibility of knockout animals, which have been very useful in studies of immune responses to infection. The vaginal epithelium in the genital tract undergoes significant hormonal changes during the menstrual cycles, and both susceptibility to HSV-2 and the nature of the induced immune responses are regulated and affected by sex hormones (Kaushic et al., 2011). Mice are more susceptible to vaginal HSV-2 infection during pregnancy and during the diestrus stage of the murine estrus cycle, when progesterone levels are the highest (Overall et al., 1975; Baker and Plotkin, 1978; Gallichan and Rosenthal, 1996). Pre-treatment of mice with Depo-Provera, a long-lasting commercial progesterone induces the diestrus stage and increases susceptibility to vaginal HSV-2 infection by 100-fold (Parr et al., 1994; Kaushic et al., 2003).
During the progression of intra-vaginal (i.vag.) HSV-2 infection in mice, the virus initially infects the vaginal epithelial cells in patches that involve the full thickness of the epithelium layer, and the underlying stroma is usually free of infection. The infected epithelial cells are shed off into the vaginal lumen (apical side) and infect the rest of the epithelium. The virus can spread horizontally within the epithelial layers to the epidermis and hair follicles, which results in loss of hair and development of skin lesions (Parr and Parr, 2003; Zhao et al., 2003). Vaginal HSV-2 infection and the resulting replication of the virus seem to be restricted to the vaginal epithelium, with no spread via viremia, as the virus generally cannot be isolated from systemic organs, blood or lymph nodes upon genital HSV infection (Overall et al., 1975; Renis et al., 1976; Podlech et al., 1996; Zhao et al., 2003). HSV-2 reaches dorsal root ganglia (DRG) via sensory neurons that innervate the site of infection, and from there the virus can spread further to the lumbar part of the spinal cord, brain stem and finally the brain (Renis et al., 1976; Georgsson et al., 1987; Podlech et al., 1996; Parr and Parr, 2003). HSV-2 can also spread to parasympathetic neurons via Para-cervical ganglia (major autonomic ganglia) of the bladder and rectum, which can cause retention of urine and feces (Parr and Parr, 2003).
Materials and Reagents
Note: All of the items mentioned in section “Materials and Reagents” can be ordered from any qualified company.
- Pipette tips
- Tissue culture plates (58 x 15 mm) (Thermo Fisher Scientific)
- Super Frost+ slides (Thermo Fisher Scientific)
- TC flask T25 (SARSTEDT)
- 70 µm pore size mesh (BD Falcon)
- 40 µm pore size mesh (BD Falcon)
- 96-well flat bottom plates (NUNC)
- 96-well round bottom plates (NUNC)
- Safety lock Eppendorf tubes
- Stainless steel beads, 5 mm (QIAGEN)
- Tissue processing/embedding cassettes (Sigma-Aldrich)
- Scalpels (Swann Morton)
- Flow-count beads (Beckman Coulter)
- Vero cells
- L929 cells
- Mouse strain: C57BL/6 mice
Notes:- The genetic background of different mouse strains can influence studies and it has been observed that different mouse strains, C57BL/6, BALB/c, SJL/J, PL/J and A/J have different susceptibilities to HSV. C57BL/6 and BALB/c mice are moderately susceptible to HSV, whereas A/J, PL/J and SJL/J mice strains are highly susceptible (Lopez, 1975; Kastrukoff et al., 2012). It has also been observed that 129Sv background mice produce higher amounts of type I and type III IFN compared to C57BL/6 in response to genital HSV-2 infection, nevertheless no difference in viral titer is observed in the vagina after HSV-2 infection (Ank, 2008).
- The vaginal HSV-2 infection model is based on pre-treatment with depo-provera. The pre-treatment with progesterone is an artificial intervention but is required for a reproducible enhanced susceptibility to HSV-2 infection in C57BL/6 mice.
- The genetic background of different mouse strains can influence studies and it has been observed that different mouse strains, C57BL/6, BALB/c, SJL/J, PL/J and A/J have different susceptibilities to HSV. C57BL/6 and BALB/c mice are moderately susceptible to HSV, whereas A/J, PL/J and SJL/J mice strains are highly susceptible (Lopez, 1975; Kastrukoff et al., 2012). It has also been observed that 129Sv background mice produce higher amounts of type I and type III IFN compared to C57BL/6 in response to genital HSV-2 infection, nevertheless no difference in viral titer is observed in the vagina after HSV-2 infection (Ank, 2008).
- Virus strain: HSV-2 333 strain (laboratory isolate, Stanberry et al., 1982)
- Vesicular stomatitis virus (VSV/V10) (Indiana Strain) (ATCC, catalog number: VR-158 )
- Phosphate buffered saline solution (PBS) (Sigma-Aldrich)
- Depo-Provera (Pfizer)
- Isoflourane (Piramal Critical Care)
- DMEM (with no supplement of antibiotics or FSC) (Lonza)
- Penicillin-streptomycin (Thermo Fisher Scientific)
- Fetal calf serum (FCS) (In Vitro Technologies)
- 0.2% human immunoglobulin (ZLB Behring)
- 0.03% methylene blue (Sigma-Aldrich)
- 2% formaldehyde (Sigma-Aldrich)
- Ethanol (Sigma-Aldrich)
- Xylene (Sigma-Aldrich)
- Methanol (Sigma-Aldrich)
- 0.5% hydrogen peroxide (H2O2) (Sigma-Aldrich)
- 10 mM Tris
- 0.5 mM EGTA (pH 9.0)
- 50 mM NH4Cl
- Polyclonal rabbit anti-HSV-2 (Agilent Technologies, Dako, catalog number: GA521 ) (1:100)
- HRP-conjugated secondary antibody (Agilent Technologies, Dako, catalog number: P044801-2 ) (1:200)
- 3,3’-diaminobenzidine (Kem-En-Tec)
- Mayer’s or Harris’s hematoxylin solution (Sigma-Aldrich)
- Eukitt reagent (Eukitt, O. Kindler)
- Collagenase/dispase (1 mg/ml) (Roche Diagnostics)
- DNase 1 (2 mg/ml) (Roche Diagnostics)
- Ethylenediaminetetraacetate acid disodium salt (EDTA) (0.02%)
- Anti-mouse CD16/CD32 Ab (eBioscience)
- IgG, 1 g/ml (Jackson ImmunoResearch)
- Anti-mouse antibodies (BD Pharmingen):
- CD45-APC (clone 30-F11)
- NK1.1-FITC (clone PK136)
- Nk1.1-PE (clone PK136)
- CD11b-PE (clone M1/70)
- Ly-6g-APC (clone 1A8)
- APC RAT IgG2b, κ Isotype
- FITC RAT IgG2a, κ Isotype
- PE RAT IgG2a, κ Isotype
- 7-AAD (Live/dead cell marker)
- CD45-APC (clone 30-F11)
- TRIzol (Invitrogen)
- DEPC water
- Chloroform (Sigma-Aldrich)
- Isopropanol
- Invitrogen Ambion DNA free kit
- Bovine serum albumin (BSA) (Sigma-Aldrich)
- 0.05% saponin (Thermo Fisher Scientific)
- 0.2% gelatin (Thermo Fisher Scientific)
- 0.3% Triton X-100 (Thermo Fisher Scientific)
- ELISA kits (R&D Systems)
- RNase free water (Roche Diagnostics)
- Oligo (dT) (Roche Diagnostics)
- Expand reverse transcriptase (Roche Diagnostics)
- Buffer A (see Recipes)
- Buffer B (see Recipes)
- Buffer C (see Recipes)
- FACS buffer (see Recipes)
Equipment
- Pipettes
- 8 arm multipipette
- Microtome (Leica)
- Microwave oven
- Rocking platform
- Humidity chamber
- Mortar/pestle
- Centrifuge
- UV-light
- Light microscopy
- Homogenizer/shaker
- Incubator
- qPCR platform of choice
- NanoDrop
- Flow cytometer
Procedure
- Vaginal infection
- Mice are pretreated by subcutaneous injection of 200 µl (2 mg/mice, diluted in PBS) of Depo-Provera.
- Five days later mice are anesthetized with isoflourane and carefully inoculated with a pipette intra vaginally with 20 μl HSV-2 (6.7 x 104 PFU) suspended in PBS, see Figure 1. It is important not to destroy or damage the vaginal epithelial layer during inoculation as this can interfere with the natural infection.
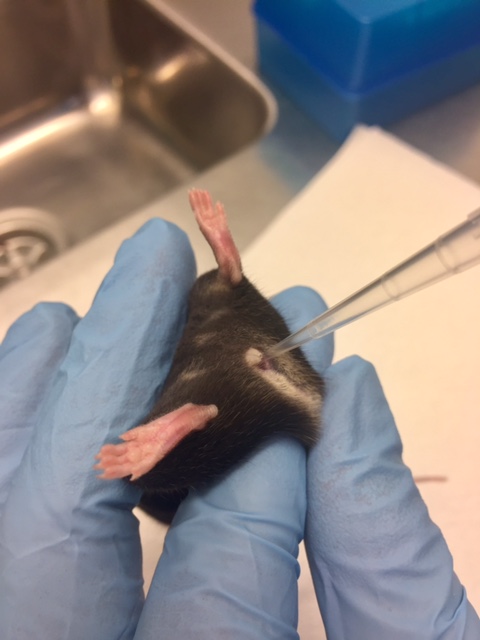
Figure 1. Inoculation of virus intravaginally with a pipette - Subsequently, the mice are placed on their back and maintained under anesthesia for 10 min.
- Vaginal washes/fluids can be collected at desired time points post infection by pipetting a volume of 40 µl PBS in and out of the vagina 12-15 times, repeated twice, and diluted to a final volume of 250 μl also in PBS. For harvest of vaginas, mice are euthanized by cervical dislocation (c.d.) and vaginas are removed surgically and used for RNA extraction, flow analysis or immunohistochemistry.
- The genitally infected animals should be observed and examined daily and scored for vaginal inflammation, neurological illness, weight loss and death. The severity of disease is scored using the following criteria: 0, healthy; 1, genital erythema; 2, moderate genital inflammation; 3, purulent genital lesion/or generally bad conditions; 4, hind limb paralysis or general very poor conditions. Mice must be sacrificed by c.d. when reaching score 4 according to institutional guidelines.
- Mice are pretreated by subcutaneous injection of 200 µl (2 mg/mice, diluted in PBS) of Depo-Provera.
- Collection of vaginal washings for measuring viral titers and cytokine production
- At desired days post infection, the vagina of the mice are washed by pipetting 2 x 40 μl of PBS in and out of the vagina, which is then further diluted in PBS to a final volume of 250 μl per mouse. Be careful not to damage vaginal epithelial layer.
- Mice are anesthetized with isoflurane during the washing procedure. Viral titers and cytokine concentrations in the vaginal wash are usually highest at day 2 post infection.
- After the washing, mice can be sacrificed for harvest of whole vaginal tissue for other analysis or continued for survival or disease score recordings.
- At desired days post infection, the vagina of the mice are washed by pipetting 2 x 40 μl of PBS in and out of the vagina, which is then further diluted in PBS to a final volume of 250 μl per mouse. Be careful not to damage vaginal epithelial layer.
- Virus plaque assay
- Place vaginal washes immediately on dry ice and store at -80 °C until analysis.
- Virus titers of the vaginal washes are determined on monolayers of Vero cells. Cells are seeded in DMEM supplemented with 5% FCS at a density of 1.2 x 106 cells/tissue culture plate (58 x 15 mm) and left overnight to settle.
- The next day the media is discarded and 400 µl fresh DMEM with 5% FCS is added to each tissue culture plate. Subsequently add 100 μl of vaginal washes per culture plate diluted in 10-fold serial dilutions in DMEM with 2% FCS so the total volume is 500 µl in each culture plate. Each culture plate represents one dilution of a vaginal wash. Make duplicates of each dilution.
- Incubate cells for 1 h and rock the plates every 15 min to ensure even distribution of the virus. After 1 h 5 ml of DMEM 5% FCS supplemented with 0.2% human immunoglobulin is added.
- The tissue culture plates are then further incubated for 2-3 days and stained with 0.03% methylene blue in order to count the plaques.
- Place vaginal washes immediately on dry ice and store at -80 °C until analysis.
- Immunohistochemistry (IHC)
Note: Paraffin is preferred in contrast to frozen sections in order to preserve morphology. We do not use perfusion fixation because the blood circulation in the vagina seems to be low. The vaginas are instead surgically removed and fixed by immersion in 2% formaldehyde in PBS for 24 h. Work at room temperature if nothing else is stated.- Vaginas are dissected and placed in 2-4% formaldehyde in PBS and fixed for 24 h.
- The fixed tissue is placed in tissue processing/embedding cassettes.
- Dehydration of fixed tissue: 70% ethanol for 2 h, 96% ethanol for 2 h and 99% for 2 h.
- The dehydrated tissue is then placed in xylene for 2 h.
- The tissue is embedded in warm (60 °C) paraffin for 2 h.
- Cut two-micrometer sections from tissue blocks on a microtome and mount the sections on Super Frost+ slides.
- The slides are the dewaxed in xylene 2 x for 4-5 min.
- Sections are rehydrated in a series of ethanol: 3 x 99% ethanol (30 min in total), 2 x 96% ethanol (20 min in total) and finally 10 min in 70% ethanol. Then the sections are rinsed in MilliQ water 3-4 times.
- Endogenous peroxidase enzymes are blocked using absolute methanol with 0.5% hydrogen peroxide (H2O2) for 10-15 min.
- Sections are boiled in microwave oven in 10 mM Tris, 0.5 mM EGTA (pH 9.0) to reveal antigens. The boiling time depends on the microwave oven ~6 min. full power and then 10 min on half power. This will provide a soft interval-boiling.
- Cool down on ice using a rocking platform.
- The free aldehyde groups are blocked by 50 mM NH4Cl in PBS for 30 min.
- Sections are then washed 3 times (3 x 10 min) in buffer A (see Recipes) and incubated with primary antibody against HSV-2 diluted in buffer B (see Recipes) overnight at 4 °C in a humidity chamber.
- The next day take the sections out of the fridge and allow to reach room temp.
- Wash with buffer C (see Recipes) (3 x 10 min.) and incubate the sections with HRP-conjugated secondary antibody (P448, 1:200) in buffer B for 1 h.
- Rinse in buffer C 3 x (total 30 min).
- Antibody binding is visualized by use of 3,3’-diaminobenzidine (DAB) (Kem-En-Tec) in 0.03% H2O2. DAB is freshly made by dissolving 1 tablet in 10 ml MilliQ water and just before use, add 10 µl 35% H2O2.
- Rinse in PBS 3 x
- The sections are counterstained with Mayer’s or Harris’s hematoxylin solution for 2 min and rinsed for 20 min under running tap water.
- The sections are mounted using Eukitt reagent.
- Vaginas are dissected and placed in 2-4% formaldehyde in PBS and fixed for 24 h.
- Flow cytometry
- Single cell suspension of mouse vagina can be obtained by enzymatic and mechanic processing. The dissected vaginas are cut in to small pieces with a scalpel and transferred to a small culture flask (T25). Add 4 ml collagenase/dispase (stock: 1 mg/ml) and 2 ml DNase I (stock: 2 mg/ml) in total 6 ml and incubate under constant stirring at 37 °C for 45 min.
- The tissue suspension is then gently mashed using a mortar pestle and filtered through a 70 µm pore size mesh and then passed through a 40 µm pore size mesh (if cells of interest are smaller than 40 µm) and washed with PBS with 0.02% EDTA to stop enzyme activity.
- Cells are then centrifuged for 8 min at 300 x g and re-suspended in 200 μl PBS/vagina. The following staining can be done in a round bottom 96-well plate.
- Prior to staining the cells are blocked for 10 min on ice with anti-mouse CD16/CD32 Ab (1:100) and mouse and rat IgG (1 µg/ml).
- Wash with cold PBS and centrifuge the plate at 300 x g for 4 min at 4 °C.
- Stain for LiveDead (7-AAD) (1:1,000 in PBS). Incubate for 15 min on ice.
- Wash twice with PBS.
- The cells are incubated with primary antibodies in 100 µl for 30 min on ice in the dark and washed in FACS buffer (see Recipies).
- The following primary anti-mouse antibodies can be used for flow identification of cell populations: CD45-APC (clone 30-F11), NK1.1-FITC (clone PK136), Nk1.1-PE (clone PK136), CD11b-PE (clone M1/70), Ly-6g-APC (clone 1A8), APC RAT IgG2b, κ Isotype, FITC RAT IgG2a, κ Isotype and PE RAT IgG2a, κ Isotype and 7-AAD (Live/dead cell marker). Flow-count beads can be added to the flow samples just before analysis for determining cell counts.
- The cells are resuspended and fixed in 250 μl 1% formaldehyde in PBS.
- Single cell suspension of mouse vagina can be obtained by enzymatic and mechanic processing. The dissected vaginas are cut in to small pieces with a scalpel and transferred to a small culture flask (T25). Add 4 ml collagenase/dispase (stock: 1 mg/ml) and 2 ml DNase I (stock: 2 mg/ml) in total 6 ml and incubate under constant stirring at 37 °C for 45 min.
- Detection of cytokines in vaginal washes
Note: In order to determine the amount of cytokines in the vaginal washes, we utilized ELISA and Luminex technology and for detection of IFN-α/β we used an L929 cell-based bioassay. Cytokines CXCL10, CXCL9, CCL5 (Rantes), IL-6, IFN-λ and IFN-γ can be easily detected by ELISA kits from any supplier. IFN-α/β bioactivity can be measured by use of an L929 cell-based bioassay in a 96-well plate.- Prior to use HSV-2 in the vaginal samples must be inactivated on ice by UV-light treatment for 6 min.
- Add 50 µl DMEM 5% FCS to each well in a flat bottom 96-well plate. Each plate can hold 8 different samples/controls (A2-H2) as the samples are diluted across the plate. Column 1 (A1-H1) serves as a cell control and column 12 (A12-H12) as a virus control.
- Add 50 µl of vaginal washes or murine IFN-α/β to each well in row 2 (A2-H2). Make successive two-fold dilutions with an 8 arm multipippette down to row 11 (A11-H11).
- L929 cells (2 x 104 cells/well in 100 µl) in DMEM supplemented with 5% FCS are added to each well and the plates incubated overnight at 37 °C. L929 cells are grown in DMEM supplemented with 200 IU/ml penicillin, 200 µg/ml streptomycin and 5% FCS.
- The next day 50 µl of Vesicular Stomatitis Virus (VSV/V10) (1.6 x 104 PFU/ml) is added to each well EXCEPT row 1 (A1-H1) (serve as a cell control) and the plates are further incubated for 2-3 days.
- If there is IFN-α/β in the samples being tested the IFN-α/β will protect the cells against the cytotoxic effect of VSV as observed in the bright field microscope. The dilution mediating 50% protection from virus induced cell death is defined as 1 U of IFN-α/β/ml. The bioassay has a lower detection limit of 6 U/ml.
- Prior to use HSV-2 in the vaginal samples must be inactivated on ice by UV-light treatment for 6 min.
- RNA extraction from tissue and quantitative Real-Time PCR
Total RNA from isolated vaginas is extracted with TRIzol, according to the recommendations of the manufacturer.- Set the centrifuge with rotor for Eppendorf tubes at 2-3 °C.
- Add 100-150 µl DEPC water and a 5 mm steel beads to each sample/vagina and homogenize the tissue using the homogenizer/shaker. Use safety lock Eppendorf tubes. Repeat if needed.
- After homogenization immediately place the samples on ice.
- Add 500 µl TRIzol to each sample and vortex. (According to Invitrogen use 1 ml TRIzol to 50-100 mg of tissue). Incubate at RT for 5 min.
- Add 1:5 chloroform (100 µl in case of 500 µl TRIzol) and vortex until the mixture is ‘candyfloss’ colored. Incubate for 5 min at RT or until two phases can be detected.
- Centrifuge for 15 min at 10,000 x g at 4 °C.
- Pipet the upper clear phase to new Eppendorf tubes. Avoid the white protein film on the side of the tubes.
- Add isopropanol (1:1). Vortex
- Place the tubes at -20 °C for 2 h or at RT for 10 min.
- Centrifuge for 12 min at 10,000 x g at 4 °C.
- Discard the supernatant. The RNA precipitate forms a white gel-like pellet at the bottom of the tube.
- Add 0.5 ml ice-cold ethanol (80 %) and vortex briefly. Centrifuge for 4 min at 10,000 x g. Repeat wash step.
- Discard the supernatant. Next, air-dry the pellet. If needed use a heating block at 80 °C.
Note: It can be difficult to dissolve the RNA pellet. - Pellet is re-suspended in 20-50 µl DEPC-water. Vortex.
- DNase-free treatment: (Invitrogen Ambion DNA free kit)
- Add 0.1 volume 10x DNase I buffer and 1 µl rDNase I to the RNA, and mix gently. Incubate at 37 °C for 20-30 min.
- Re-suspend DNase Inactivation Reagent and add 0.1 volume (min. 2 µl) to each sample and mix.
- Incubate the samples for 2 min at RT. Mix occasionally. Prepare new Eppendorf tubes.
- Centrifuge for 1.5 min at 10,000 x g at 4 °C.
- Transfer the suspensions to new Eppendorf tubes. Store the samples at -80 °C.
Notes:
- The RNA concentration can be measured by use of a NanoDrop, A260 for nucleic acid and 280 for protein.
- For RNA the 260/280 ratio ≈ 2 is considered pure.
- Set the centrifuge with rotor for Eppendorf tubes at 2-3 °C.
Recipes
- DMEM supplemented with 200 IU/ml penicillin, 200 µg/ml streptomycin and FCS
- DMEM supplemented with 2% FCS and 0.2% human immunoglobulin
- Buffer A
PBS with 1% BSA, 0.05% Saponin, 0.2% gelatin - Buffer B
PBS with 0.1% BSA and 0.3% Triton X-100 - Buffer C
PBS with 0.1% BSA, 0.2% gelatin and 0.05% Saponin - FACS buffer
2 % BSA in PBS (0.09% Azide if necessary)
Acknowledgments
We thank K.S. Petersen and I.M. Poulsen for technical assistance and the AU FACS Core facility for technical help. Supported by The Danish Medical Research Council (12-124330 to S.R.P.; 1331-xg00133B to H.H.W.), the Novo Nordisk Foundation (S.R.P.), the Lundbeck Foundation (R34-3855 to S.R.P.), Aarhus University Research Foundation (S.R.P), Faculty of Health Sciences, AU (M.B.I.), the Danish National Research Foundation (DNRF107 to H.H.W.), the Excellence Program for Interdisciplinary Research (CDO2016 to H.H.W.).
References
- Ank, N., Iversen, M. B., Bartholdy, C., Staeheli, P., Hartmann, R., Jensen, U. B., Dagnaes-Hansen, F., Thomsen, A. R., Chen, Z., Haugen, H., Klucher, K. and Paludan, S. R. (2008). An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol 180(4): 2474-2485.
- Baker, D. A. and Plotkin, S. A. (1978). Enhancement of vaginal infection in mice by herpes simplex virus type II with progesterone. Proc Soc Exp Biol Med 158(2): 131-134.
- Gallichan, W. S. and Rosenthal, K. L. (1996). Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 224(2): 487-497.
- Georgsson, G., Martin, J. R., Stoner, G. L. and Webster, H. F. (1987). Virus spread and initial pathological changes in the nervous system in genital herpes simplex virus type 2 infection in mice. A correlative immunohistochemical, light and electron microscopic study. Acta Neuropathol 72(4): 377-388.
- Kastrukoff, L. F., Lau, A. S. and Thomas, E. E. (2012). The effect of mouse strain on herpes simplex virus type 1 (HSV-1) infection of the central nervous system (CNS). Herpesviridae 3: 4.
- Kaushic, C., Ashkar, A. A., Reid, L. A. and Rosenthal, K. L. (2003). Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol 77(8): 4558-4565.
- Kaushic, C., Nazli, A., Ferreira, V. H. and Kafka, J. K. (2011). Primary human epithelial cell culture system for studying interactions between female upper genital tract and sexually transmitted viruses, HSV-2 and HIV-1. Methods 55(2): 114-121.
- Kaushic, C., Roth, K. L., Anipindi, V. and Xiu, F. (2011). Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. J Reprod Immunol 88(2): 204-209.
- Lopez, C. (1975). Genetics of natural resistance to herpesvirus infections in mice. Nature 258(5531): 152-153.
- Nahmias, A. J., London, W. T., Catalano, L. W., Fuccillo, D. A., Sever, J. L. and Graham, C. (1971). Genital herpesvirus hominis type 2 infection: an experimental model in cebus monkeys. Science 171(3968): 297-298.
- Overall, J. C., Jr., Kern, E. R., Schlitzer, R. L., Friedman, S. B. and Glasgow, L. A. (1975). Genital herpesvirus hominis infection in mice. I. Development of an experimental model. Infect Immun 11(3): 476-480.
- Parr, M. B., Kepple, L., McDermott, M. R., Drew, M. D., Bozzola, J. J. and Parr, E. L. (1994). A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest 70(3): 369-380.
- Parr, M. B. and Parr, E. L. (2003). Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J Neurovirol 9(6): 594-602.
- Podlech, J., Hengerer, F., Fleck, M., Walev, I. and Falke, D. (1996). Replication of herpes simplex virus type 1 and 2 in the medulla of the adrenal gland after vaginal infection of mice. Arch Virol 141(10): 1999-2008.
- Renis, H. E. (1977). Chemotherapy of genital herpes simplex virus type 2 infections of female hamsters. Antimicrob Agents Chemother 11(4): 701-707.
- Renis, H. E., Eidson, E. E., Mathews, J. and Gray, J. E. (1976). Pathogenesis of herpes simplex virus types 1 and 2 in mice after various routes of inoculation. Infect Immun 14(2): 571-578.
- Roizman, B., Knipe, D. M. and Whitley, R. J. (2013). Chap. 60: Herpes Simplex Viruses. In: Knipe, M. D. (Eds). Fields Virology. 6th edition. Lippincott Williams & Wilkins 1823-1897.
- Stanberry, L. R., Kern, E. R., Richards, J. T., Abbott, T. M. and Overall, J. C., Jr. (1982). Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis 146(3): 397-404.
- Zhao, X., Deak, E., Soderberg, K., Linehan, M., Spezzano, D., Zhu, J., Knipe, D. M. and Iwasaki, A. (2003). Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med 197(2): 153-162.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Iversen, M. B., Paludan, S. R. and Holm, C. K. (2017). Vaginal HSV-2 Infection and Tissue Analysis. Bio-protocol 7(13): e2383. DOI: 10.21769/BioProtoc.2383.
Category
Immunology > Mucosal immunology > Genitourinary tract > Cell isolation
Microbiology > in vivo model > Viruses
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








