- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Formaldehyde Fixation of Extracellular Matrix Protein Layers for Enhanced Primary Cell Growth
Published: Vol 7, Iss 13, Jul 5, 2017 DOI: 10.21769/BioProtoc.2374 Views: 9186
Reviewed by: Yanjie LiHui ZhuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Accurate Identification of Cell Cycle Stages in RPE1 Cells Using the ImmunoCellCycle-ID Method
Syon Reddy [...] Aussie Suzuki
Aug 5, 2025 1870 Views

Spheroid Sheets: A Scalable Platform for Producing Tissue Membrane Constructs
Quang Bach Le [...] Deepak Choudhury
Nov 20, 2025 1536 Views

A Protocol to Induce Brown and Beige Adipocyte Differentiation From Murine and Human Adipose-Derived SVF
Rohit Raj Yadav [...] Narendra Verma
Dec 5, 2025 1642 Views
Abstract
Coating tissue culture vessels with the components of the extracellular matrix such as fibronectin and collagens provides a more natural environment for primary cells in vitro and stimulates their proliferation. However, the effects of such protein layers are usually rather modest, which might be explained by the loss immobilized proteins due to their weak non-covalent association with the tissue culture plastic. Here we describe a simple protocol for a controlled fixation of fibronectin, vitronectin and collagen IV layers by formaldehyde, which substantially enhances the stimulation of primary cell proliferation by these extracellular proteins.
Keywords: Mesenchymal stem cellsBackground
The components of the extracellular matrix (ECM) such as fibronectin, laminin, vitronectin and collagens are often used for coating tissue culture vessels since they provide a more natural environment for primary cells in vitro and stimulate their proliferation (Sawada et al., 1987; Rajaraman et al., 2013). However, the observed stimulation of cell proliferation by these protein layers is usually fairly modest. This might be explained by their weak non-covalent association with the tissue culture plastic resulting in delamination and loss of immobilized protein molecules. Recently, it has been shown that the retention of ECM produced by the cells might be significantly increased by covalent immobilization of fibronectin to the plastic surface (Prewitz et al., 2013). However, ECM production is cumbersome and may be difficult to standardize. We have demonstrated that simple formaldehyde fixation under controlled conditions of layers formed by the selected individual ECM proteins can substantially enhance positive effects of these proteins on cell proliferation (Andreeva et al., 2016). Here we describe a detailed protocol of culture plastic coating and formaldehyde fixation for three individual components of ECM, namely fibronectin, vitronectin and collagen IV. Although we did not test the effects of controlled fixation with other protein constituents of the ECM, the positive results obtained with our protocol for three proteins of vastly differing molecular and biological properties provide sufficient reasons to assume the general applicability of the described procedure for enhancing proliferation stimulatory properties of a wider range of ECM proteins.
Materials and Reagents
- 3.5 cm cell culture dish (Greiner Bio One International, CELLSTAR®, catalog number: 627160 )
- Sterile pipette filter tips 200 and 1,000 μl (Greiner Bio One International, catalog numbers: 739288 and 740288 , respectively)
- Parafilm M (Sigma-Aldrich, catalog number: P7668 )
- 15 ml centrifuge tube (Greiner Bio One International, CELLSTAR®, catalog number: 188261 )
- 50 ml centrifuge tube (Greiner Bio One International, CELLSTAR®, catalog number: 227261 )
- 1.8 ml round bottom cryogenic tubes (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 375418 )
- 0.22 μm syringe filter (GVS, catalog number: 1213641 )
- 10 and 20 ml syringes (SFM Hospital Products, catalog numbers: 534235 and 534236 , respectively)
- 2 and 10 ml Serological pipets (Greiner Bio One International, CELLSTAR®, catalog numbers: 710180 and 607107 , respectively)
- Phosphate-buffered saline (PBS), pH 7.4 tablets (Thermo Fisher Scientific, GibcoTM, catalog number: 18912014 )
- Paraformaldehyde (Sigma-Aldrich, catalog number: 76240 )
Note: This product has been discontinued. - Sodium hydroxide (NaOH), 10 M (Sigma-Aldrich, catalog number: 72068 )
- Fibronectin human (Imtek, catalog number: H Fne-C )
- Vitronectin human (Imtek, catalog number: H Vne-C )
- Collagen IV bovine (Imtek, catalog number: B C44-C )
- Acetic acid (Sigma-Aldrich, catalog number: 695092 )
- Ethanol 96% (Sigma-Aldrich, catalog number: 24105 )
Note: This product has been discontinued. - Sterile distilled water
- 1x phosphate-buffered saline (PBS) pH 7.4 (see Recipes)
- Formaldehyde solution (see Recipes)
- Human fibronectin solution (prepare freshly) (see Recipes)
- Human vitronectin solution (prepare freshly) (see Recipes)
- Bovine collagen IV solution (prepare freshly) (see Recipes)
Equipment
- Pipette controller (Corning, catalog number: 4091 )
- Automatic single-channel pipettes, 20-200 and 100-1,000 μl (Gilson-compatible)
- Magnetic hot plate stirrer (Sigma-Aldrich, catalog number: Z168580 )
- Magnetic stirring bar, 7 x 2 mm (Fisher Scientific, FisherbrandTM, catalog number: 14-513-63 )
- Laminar flow tissue culture hood
Note: This item can be ordered from any qualified company. - Refrigerator with interior power outlets
Note: This item can be ordered from any qualified company. - CO2 incubator (Sanyo, catalog number: MCO-18AIC )
Note: This product has been discontinued. Possible substitute: Panasonic Healthcare, model: MCO-18AC. - Centrifuge 5810 R (Eppendorf, model: 5810 R , catalog number: 5811000320)
- Autoclave
Note: This item can be ordered from any qualified company.
Procedure
- Coating Petri dishes with fibronectin followed by formaldehyde fixation
Note: All procedures below should be performed under a laminar flow hood.- Add 1 ml of the 50 µg/ml fibronectin solution (see Recipes) to each of the 3.5-cm Petri dishes. Make sure the entire dish surface is completely covered by gently swirling the solution or redistributing it over the surface using pipette tip.
Note: The fibronectin layer is only about 1-mm deep for the indicated volume, thus it is recommended to perform this operation with Petri dishes placed on a strictly horizontal surface to ensure even coverage of all areas of the dish. - Incubate dishes with the lid on for 1.5 h at 37 °C in a humidified CO2 incubator.
- Aspirate carefully and save the fibronectin solution.
Note: The saved fibronectin solution can be re-used at least once. Store it at 4 °C and use within one week. - Add 1 ml of 0.4% formaldehyde solution (see Recipes). Incubate dishes with the lid on for 20 min under a sterile laminar flow hood.
- Carefully aspirate the formaldehyde solution, wash twice with 2 ml of sterile PBS and then 4 times with 4 ml of sterile PBS.
Note: Formaldehyde solution should be disposed of as a hazardous waste. - Dry dishes for 1 h under the laminar flow hood.
Note: Fibronectin-covered dishes can be sealed with Parafilm and stored at 4 °C for up to 1 week before use.
- Add 1 ml of the 50 µg/ml fibronectin solution (see Recipes) to each of the 3.5-cm Petri dishes. Make sure the entire dish surface is completely covered by gently swirling the solution or redistributing it over the surface using pipette tip.
- Coating Petri dishes with vitronectin followed by formaldehyde fixation
Note: All procedures below should be performed under a laminar flow hood.- Add 0.5 ml of the 10 µg/ml vitronectin solution (see Recipes) to each of the 3.5-cm Petri dishes. Make sure the entire dish surface is completely covered by gently swirling the solution or redistributing it over the surface using pipette tip.
Note: The vitronectin layer is only about 0.5-mm deep for the indicated volume, thus it is recommended to perform this operation with Petri dishes placed on a strictly horizontal surface to ensure an even coverage of all areas of the dish. - Incubate dishes with the lid on for 1.5 h at 37 °C in a humidified CO2 incubator.
- Aspirate carefully and save the vitronectin solution.
Note: The saved vitronectin solution can be re-used at least once. Store it at 4 °C and use within one week. - Add 1 ml of 0.4% formaldehyde solution (see Recipes). Incubate dishes with the lid on for 20 min under a sterile laminar flow hood.
- Carefully aspirate the formaldehyde solution, wash twice with 2 ml of sterile PBS and then 4 times with 4 ml of sterile PBS.
Note: Formaldehyde solution should be disposed of as a hazardous waste. - Dry dishes for 1 h under the laminar flow hood.
Note: Vitronectin-covered dishes can be sealed with Parafilm and stored at 4 °C for up to 1 week before use.
- Add 0.5 ml of the 10 µg/ml vitronectin solution (see Recipes) to each of the 3.5-cm Petri dishes. Make sure the entire dish surface is completely covered by gently swirling the solution or redistributing it over the surface using pipette tip.
- Сoating Petri dishes with collagen IV followed by formaldehyde fixation
Note: Coating Petri dishes overnight is performed in the refrigerator, the rest of procedures should be performed under a laminar flow hood while keeping collagen solution on ice and minimizing exposure to room temperature conditions.- Add 0.5 ml of the 50 µg/ml collagen IV solution (see Recipes) to each of 3.5-cm Petri dishes. Make sure the entire dish surface is completely covered by gently swirling the solution or redistributing it over the surface using pipette tip. Immediately transfer the dishes to the refrigerator.
- Incubate dishes with the lid on overnight at 4 °C.
Note: The collagen layer is only about 0.5-mm deep for the indicated volume, thus it is important to perform this operation with Petri dishes placed on a strictly horizontal surface to ensure even coverage of all areas of the dish. - Transfer dishes to the laminar flow hood, carefully aspirate and save collagen solution.
Note: The saved collagen solution can be re-used at least once. Store it at 4 °C and use within one week. - Add 1 ml of 0.4% formaldehyde solution (see Recipes). Incubate for 20 min under sterile laminar flow hood.
- Carefully aspirate the formaldehyde solution, wash twice with 2 ml of sterile PBS and then 4 times with 4 ml of sterile PBS.
Note: Formaldehyde solution should be disposed of as a hazardous waste. - Dry dishes for 1 h under the laminar flow hood.
Note: Fibronectin-covered dishes can be sealed with Parafilm and stored at 4 °C for up to 1 week before use.
- Add 0.5 ml of the 50 µg/ml collagen IV solution (see Recipes) to each of 3.5-cm Petri dishes. Make sure the entire dish surface is completely covered by gently swirling the solution or redistributing it over the surface using pipette tip. Immediately transfer the dishes to the refrigerator.
Data analysis
The assessment of the success of ECM protein coating and fixation includes analysis of MSC or other primary cell proliferation rate. Proliferation rates are determined by seeding 15 to 30 thousand cells per 3.5-cm dish followed by culturing for 1 or 2 days. A minimal testing setup includes: a) control untreated dishes; b) dishes, coated with ECM protein but unfixed; c) dishes coated with ECM and fixed with formaldehyde. Each variant is performed in triplicate, cells after culturing are collected by trypsinization followed by manual or automatic cell counting. It is recommended to perform counting of each cell sample two or three times using independent cell aliquots to increase the accuracy of analysis. It is important to assess the cell proliferation while the cells are within the exponential growth phase, that is, with cell confluence not exceeding 50%. The proliferation rate can be represented as an expansion factor (ratio of harvested to seeded cells) or population doublings (log2 of expansion factor). The results are analyzed by determining mean, standard deviations and performing Student’s t-tests. The typical result of such analysis is shown in Figure 1.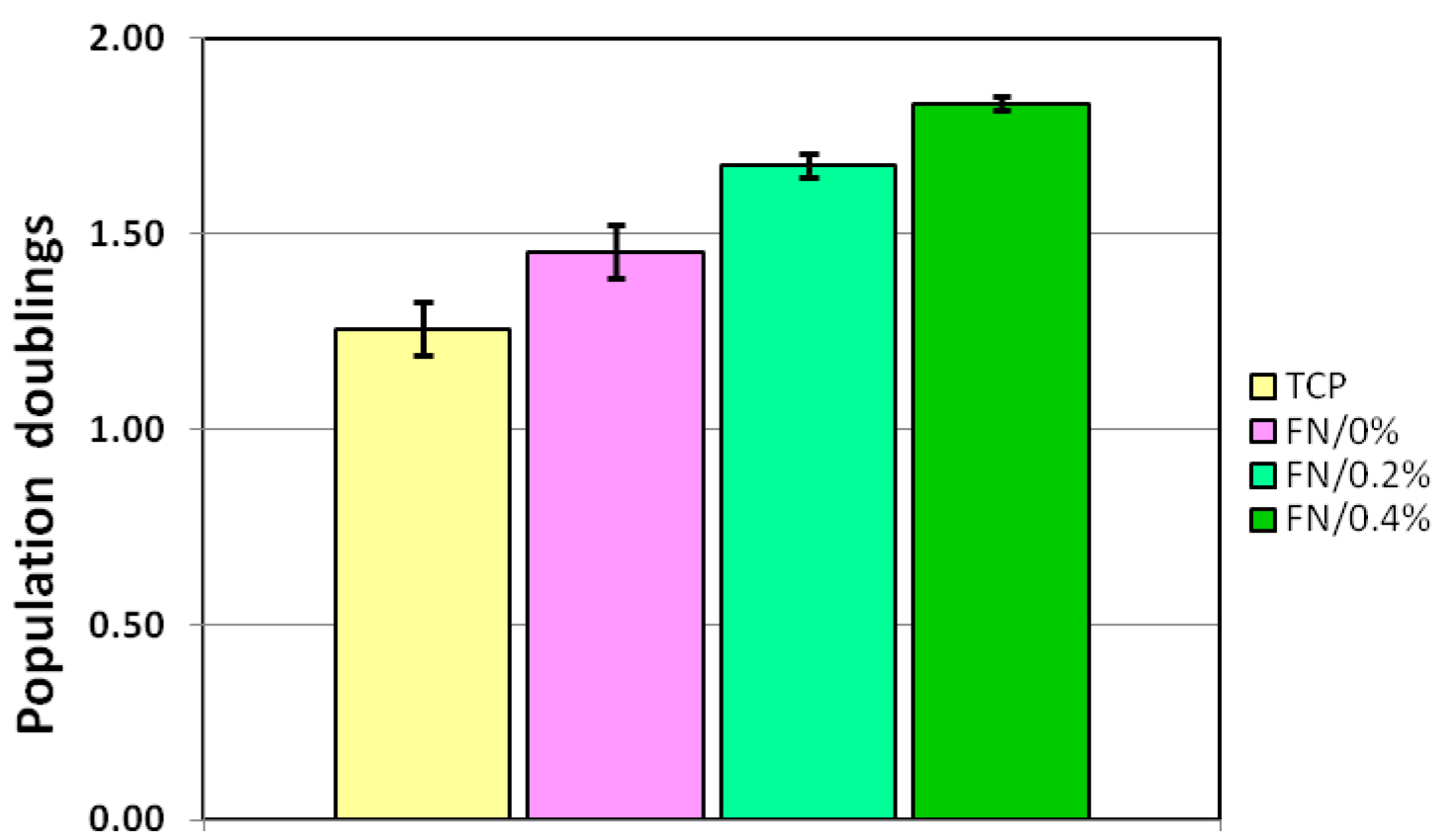
Figure 1. Controlled fibronectin fixation significantly enhances expansion of mesenchymal stem cells (MSCs). Mouse MSC growth under hypoxia conditions (5% O2) on control uncoated dishes (TCP) and dishes coated with fibronectin at a concentration of 50 µg/ml. Fibronectin-coated dishes were left unfixed (FN/0%) or fixed with 0.2% formaldehyde (FN/0.2%) or 0.4% formaldehyde (FN/0.4%). Y axis depicts the number of population doublings underwent by the culture within 24 h. Data are presented as mean ± SD.
Notes
- Imtek is a Russian company producing, among others, extracellular matrix proteins for cell culture. Their website: http://new.imtek.ru/eng. We have as yet no experience with the extracellular matrix proteins from other companies.
- The formaldehyde concentrations producing best results in our setup for 20-min fixation time are 0.3%-0.4%. Higher formaldehyde concentrations result in diminished effects on cell proliferation (Andreeva et al., 2016). When working with other extracellular matrix proteins not tested by us, it is highly advisable to perform test fixations with formaldehyde concentrations ranging from 0.2% to 0.6% to find the optimal fixation conditions.
- The measured temperature during formaldehyde fixation (i.e., under laminar flow hood) in our setup is 24 °C to 26 °C. Since the level of fixation depends on the temperature, it is recommended to determine the temperature under the laminar hood to make sure the deviation from the above temperature does not exceed 2 °C. If the temperature is out of this range, it is recommended to perform test fixations for somewhat different periods of time to determine the optimal fixation time for a given experimental setup.
- According to our results (Andreeva et al., 2016), coating Petri dishes with fibronectin at the 50 µg/ml concentration produced better results as compared to the 10 µg/ml concentration, whereas increasing fibronectin concentration to 250 µg/ml produced only a small additional effect. Although we did not test this yet, it is fairly possible that extending time for coating dishes over the 1.5-h period used in our protocol may produce a sizeable increase in the effects of fibronectin layers at 10 µg/ml concentration.
- Coating with vitronectin, due to a high cost of this protein, was performed at lower concentration (10 µg/ml) as compared to fibronectin and collagen IV (50 µg/ml), and produced smaller effects than those observed for the latter two proteins (Andreeva et al., 2016). Based on analogy with fibronectin results, it is likely that increasing concentration of vitronectin during plastic coating will produce larger effects.
- Since the treatment of plastic surfaces for different types vessels and by various manufacturers may differ, it is fairly possible that the effects of fixed extracellular matrix protein layers on cell proliferation may vary. In our hands, fibronectin coating followed by fixation was more efficient for 3.5-cm Petri dishes than for 6-well plates.
- Formaldehyde during storage may polymerize at lower temperatures and decompose at higher temperatures, thus decreasing the effective formaldehyde concentration. It is therefore recommended to use the prepared formaldehyde solution within one week and, preferably, prepare formaldehyde solution freshly for each fixation experiment.
- It is important that collagen IV is processed at low temperature to prevent its slow denaturation which occurs even at room temperature.
- Among the cell types tested by us, the most pronounced effects were observed for mesenchymal stem cells, both murine and human ones, smaller effects were obtained for fibroblasts, and no positive effects were observed for established cell lines (Andreeva et al., 2016).
Recipes
- 1x phosphate-buffered saline (PBS) pH 7.4
2 tablets PBS (10 g)
Add distilled water to a final volume of 1 L
Sterilize by autoclaving
Store at room temperature - Formaldehyde solution
Add 4.0 g paraformaldehyde to a beaker with magnetic stirring bar
Add distilled water to a final volume 100 ml
Boil until complete dissolution on a hot plate magnetic stirrer, cool down
Add 10 N NaOH dropwise to pH 7.4
Store at 5 °C, use within 1 week
Dilute prior to the experiment to the desired concentration (usually, 0.4%) with sterile 1x PBS - Human fibronectin solution (prepare freshly)
1 ml fibronectin sterile solution 1 mg/ml
19 ml sterile 1x PBS
Store at 4 °C, use within one week - Human vitronectin solution (prepare freshly)
0.25 mg sterile vitronectin dry powder
25 ml sterile 1x PBS
Store at 4 °C, use within one week - Bovine collagen IV solution (prepare freshly)
Notes:- All manipulations should be performed at temperatures 0 °C to 5 °C using pre-chilled solutions.
- Sterilize the magnet before use in 70% ethanol for 1 h, then under UV irradiation for 1 h.
- All manipulations should be performed at temperatures 0 °C to 5 °C using pre-chilled solutions.
- Add 1.0 ml of 20 mM acetic acid (filter-sterilized) to 0.5 mg sterile collagen IV dry powder in a 1.8-ml round-bottom cryovial
- Add a small-size magnetic stirring bar and stir the collagen solution overnight on a magnetic stirrer at 4 °C
- Dilute the prepared collagen IV solution 10-fold with cold sterile 20 mM acetic acid to a final concentration of 50 µg/ml
- Store the final solution at temperature 4 °C, use within one week
Acknowledgments
The protocol described herein was adapted from Andreeva et al. (2016). This work was supported by the grant (No. 14-04-01855) from the Russian Foundation for Basic Research and by the Program of fundamental research for state academies for 2013-2020 years (Task 0103-2014-0006 Subprogram #58 Molecular genetics, mechanisms of realization of genetic information, bioengineering).
References
- Andreeva, N. V., Leonova, O. G., Popenko, V. I. and Belyavsky, A. V. (2016). Controlled formaldehyde fixation of fibronectin layers for expansion of mesenchymal stem cells. Anal Biochem 514: 38-41.
- Prewitz, M. C., Seib, F. P., von Bonin, M., Friedrichs, J., Stissel, A., Niehage, C., Muller, K., Anastassiadis, K., Waskow, C., Hoflack, B., Bornhauser, M. and Werner, C. (2013). Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat Methods 10(8): 788-794.
- Rajaraman, G., White, J., Tan, K. S., Ulrich, D., Rosamilia, A., Werkmeister, J. and Gargett, C. E. (2013). Optimization and scale-up culture of human endometrial multipotent mesenchymal stromal cells: potential for clinical application. Tissue Eng Part C Methods 19(1): 80-92.
- Sawada, N., Tomomura, A., Sattler, C. A., Sattler, G. L., Kleinman, H. K. and Pitot, H. C. (1987). Effects of extracellular matrix components on the growth and differentiation of cultured rat hepatocytes. In Vitro Cell Dev Biol 23(4): 267-273.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Andreeva, N. V. and Belyavsky, A. V. (2017). Formaldehyde Fixation of Extracellular Matrix Protein Layers for Enhanced Primary Cell Growth. Bio-protocol 7(13): e2374. DOI: 10.21769/BioProtoc.2374.
Category
Biochemistry > Protein > Structure
Cell Biology > Cell viability > Cell proliferation
Stem Cell > Adult stem cell > Mesenchymal stem cell
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










