- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Primary Olfactory Ensheathing Cell Culture from Human Olfactory Mucosa Specimen
Published: Vol 7, Iss 10, May 20, 2017 DOI: 10.21769/BioProtoc.2275 Views: 8537
Reviewed by: Oneil G. BhalalaEhsan KheradpezhouhXiaoyu Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2232 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1460 Views
Abstract
The human olfactory mucosa is located in the middle and superior turbinates, and the septum of nasal cavity. Olfactory mucosa plays an important role in detection of odours and it is also the only nervous tissue that is exposed to the external environment. This property leads to easy access to the olfactory mucosa for achieving various researches. The lamina propria of olfactory mucosa consists of olfactory ensheathing cells (OECs) that cover the nerve fibers of olfactory. Here we describe a protocol for isolation of OECs from biopsy of human olfactory mucosa.
Keywords: Human olfactory mucosaBackground
Olfactory ensheathing cells (OECs) are glial cells that express various antigens similar to astrocytes and Schwann cells such as glial fibrillary-associated protein (GFAP), S100-beta, p75 low-affinity nerve growth factor receptor, vimentin, nestin, and neuropeptide Y (Singh et al., 2013). Olfactory ensheathing cells release different neurotrophic factors and adhesion molecules that function in cellular growth and adhesions of central nervous system (Pastrana et al., 2007). In addition, these cells play an important role in the regeneration of the damaged central nervous system such as treatment of spinal cord injury and neurodegenerative diseases (Novikova et al., 2011). We select OECs as research material in our study as they have several advantage properties such as high migratory capacity, accessible source, differentiation from stem cells of nasal olfactory mucosa, and non-tumorigenicity behavior (Huang et al., 2008; Escada et al., 2009). This protocol describes a step-by-step procedure for the isolation of OECs from Human Olfactory Mucosa Specimen.
Materials and Reagents
- 15 ml centrifuge tubes (Corning, Falcon®, catalog number: 352096 )
- 50 ml centrifuge tubes (Corning, Falcon®, catalog number: 352070 )
- T25 flask culture (Nest Biotechnology, catalog number: 707003 )
- 24-well plates (Nest Biotechnology, catalog number: 702001 )
- No. 10 scalpel blade surgical tool (Aspen Surgical, catalog number: 371610 )
- Cell strainer sieve (Corning, Falcon®, catalog number: 352340 )
- Petri dish culture (Nest Biotechnology, catalog number: 704001 )
- Olfactory mucosa fresh specimen (Human)
- Hanks’ balanced salt solution (HBSS) (Thermo Fisher Scientific, GibcoTM, catalog number: 24020117 )
- Antibiotic-antimycotic (Thermo Fisher Scientific, GibcoTM, catalog number: 15240062 )
- Dispase II (Sigma-Aldrich, catalog number: D4693 )
- Collagenase IA (Sigma-Aldrich, catalog number: C9891 )
- Dulbecco’s modified Eagle medium/F12 (DMEM/F12) (Thermo Fisher Scientific, GibcoTM, catalog number: 31331028 )
- Nerve growth factor (NGF) (Sigma-Aldrich, catalog number: N0513 )
- 0.25% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10270106 )
- Triton X-100 (Sigma-Aldrich, catalog number: T8787 )
- Bovine serum albumin (BSA, powder) (Sigma-Aldrich, catalog number: A2058 )
- Primary antibody of rabbit anti-S100-beta (Sigma-Aldrich, catalog number: S2644 )
- FITC-conjugated goat anti-rabbit (Abcam, catalog number: ab6717 )
- (Optional) Cytosine arabinoside or Cytarabine (Sigma-Aldrich, catalog number: C3350000 )
- Phosphate buffer saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 18912014 )
- Paraformaldehyde (powder) (Sigma-Aldrich, catalog number: P6148 )
- Culture medium (see Recipes)
- Phosphate buffer saline (PBS) (see Recipes)
- 4% paraformaldehyde (see Recipes)
- 0.2% Triton X-100 (see Recipes)
- 10% bovine serum albumin (BSA) (see Recipes)
Equipment
- Small forceps surgical tools (Fine Science Tools, catalog number: 11050-10 )
- Hemocytometer (Sigma-Aldrich, catalog number: Z359629 )
- Inverted fluorescence microscope (Optika, model: XDS-2FL )
- Centrifuge machine (Hettich Lab Technology, model: Universal 320 R )
- Ventilation hood (Vision Scientific, model: VS-7120LV )
- CO2 cell culture incubator (Memmert, model: INC108 T2T3 )
- 37 °C water bath (Memmert, model: WNB 14 )
Procedure
- Collection of olfactory mucosa tissue
- A fresh sample (diameter of 4 mm) was obtained endoscopically from the superior turbinate tissue of a patient under general anesthesia.
- This study was approved by the Ethical Commission of the Tehran University of Medical Sciences.
- Before operation, a signed written consent was given by the patient.
- There is no age limitation for providing fresh sample in this study.
- After coordination with an Ear Nose and Throat (ENT) surgeon, the olfactory mucosa tissue was placed in a 15 ml tube containing 5 ml cold HBSS with 10% antibiotics (Antibiotic-antimycotic).
- Olfactory mucosa tissue was delivered to the lab on dry ice.
- A fresh sample (diameter of 4 mm) was obtained endoscopically from the superior turbinate tissue of a patient under general anesthesia.
- Isolation of OECs from olfactory mucosa
- Wash the olfactory mucosa tissue 3 times with 5 ml cold PBS each time.
- Incubate the olfactory mucosa tissue in 1 ml dispase II solution (2.4 IU/ml), for 1 h at 37 °C.
- Dissect lamina propria from the olfactory epithelium using a micro spatula under an inverted light microscope. Lamina propria is the thick orange layer and epithelium is the thin translucent gray layer.
- Cut the lamina propria into small pieces.
- Transfer the pieces to a new 15 ml Falcon tube containing 1 ml of collagenase IA and incubate for 20 min at 37 °C.
- Inactivate collagenase IA by adding 9 ml of PBS into the tube.
- Centrifuge the suspension at 250 x g for 5 min.
- Resuspend the resulting pellet in 1 ml of Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium supplemented with neuronal growth factor (NGF, 50 ng/ml), 1% (100 U/ml) antibiotic-antimycotic.
- Transfer the suspension containing tiny pieces of tissue into two T25 flasks containing 5 ml of Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium supplemented with neuronal growth factor (NGF, 50 ng/ml), 1% (100 U/ml) antibiotic-antimycotic each.
- Incubate in an incubator at 37 °C, 5% CO2 and 95% humidity for 3-4 weeks.
- Change culture medium every 3 days.
Note: After 14 days culture of human olfactory lamina propria, OECs are released from periphery of spherical clusters. The morphology of most cells is spindle-shaped and bipolar with long processes and small cytoplasm or multipolar form with short processes and plentiful cytoplasm such as stellate morphology. Note that there may be fibroblast contamination in lamina propria culture. Fibroblast morphology is also spindle-shaped and bipolar with long processes. (Figures 1 and 2) - After 3-4 weeks, OECs are ready for subculture.
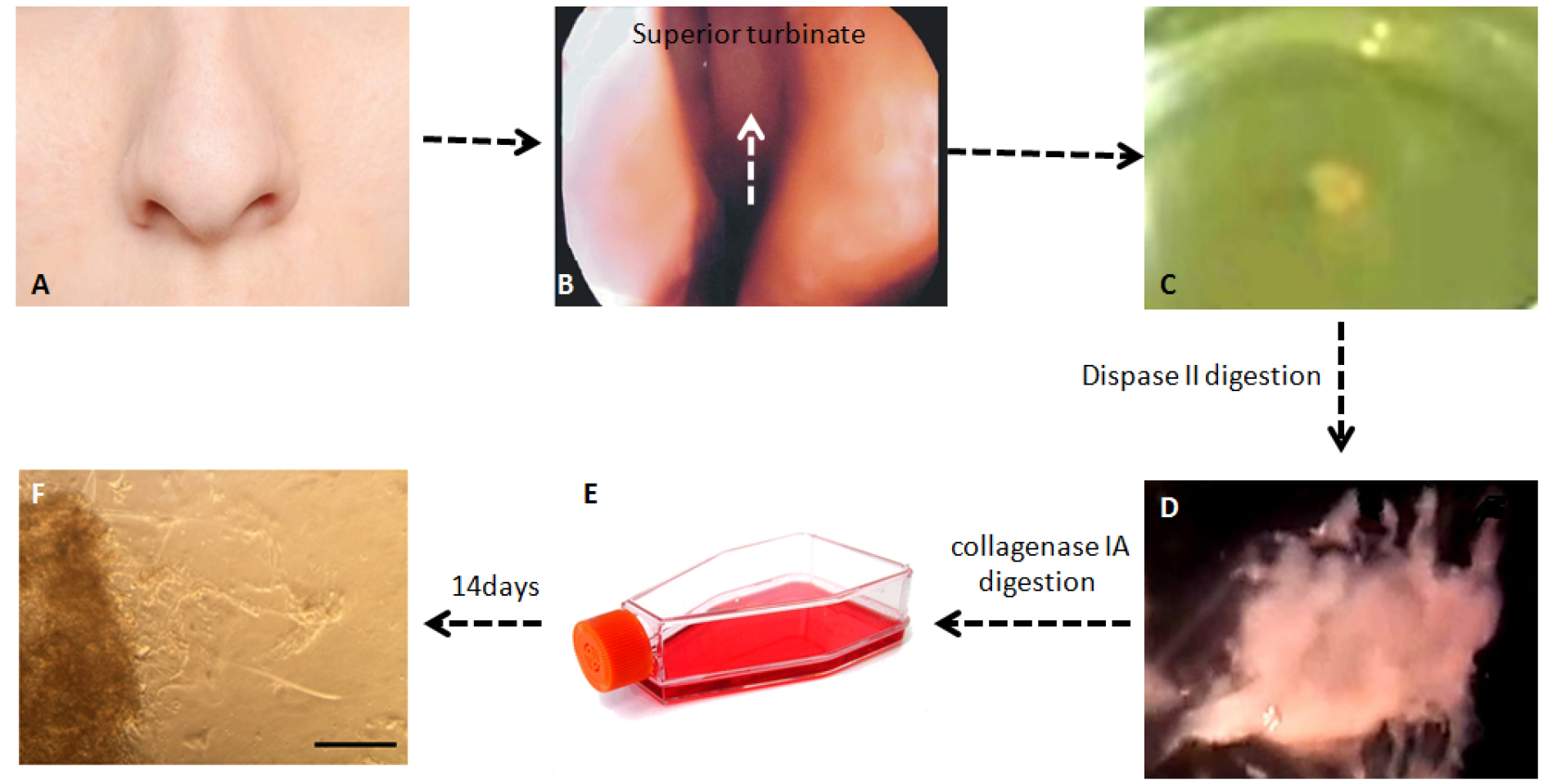
Figure 1. Different stages of OECs isolation from human olfactory mucosa. A. Outside view of nose; B. Location of superior turbinate in the olfactory mucosa; C. Human olfactory mucosa biopsy from superior turbinate; D. Lamina propria of olfactory mucosa after dispase II digestion; E. Lamina propria cultured after collagenase IA digestion; F. Olfactory ensheathing cells released from periphery of lamina propria, scale bar = 100 µm.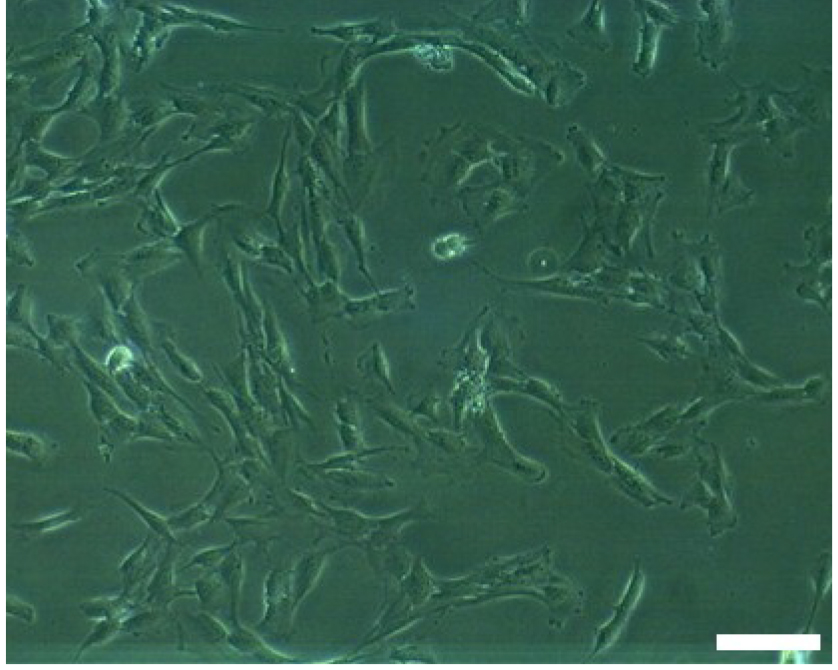
Figure 2. OECs after first passage. Scale bar = 100 µm.
- Wash the olfactory mucosa tissue 3 times with 5 ml cold PBS each time.
- Sub-culture of primary OECs of olfactory mucosa lamina propria
- Check the confluency of OECs under an inverted microscope. Subculture the cells when the cell density reaches 80% confluence 3-4 weeks after seeding.
- Remove the medium from each T25 flask under a biological hood with a 5 ml sterile pipette.
- Wash the cells with 3 ml of warm (37 °C) PBS and then remove PBS.
- Trypsinize the cells with 1 ml of warm (37 °C) 0.05% trypsin-EDTA for 1 min in an incubator at 37 °C.
- Stop trypsin-EDTA reaction by adding 4 ml of DMEM/F12 containing 10% FBS and 1% antibiotic-antimycotic into each T25 flask and then transfer the cell suspension from each flask to a 15 ml Falcon tube.
- Centrifuge the cell suspension at 250 x g for 5 min at room temperature.
- Remove the medium from each tube with a 5 ml sterile pipette.
- Resuspend the pellet in each tube with 1 ml of fresh medium containing DMEM/F12, 10% FBS and 1% antibiotic-antimycotic.
- After re-suspend and counting, seed the cells into a 24-well dish (at a density of 5 x 103 cells per well) for immunocytochemistry (go to step D2) and into T25 flasks (5 x 106 cells in flask) for subculture.
- Subculture the T25 flasks containing OECs in (DMEM)/F12 medium containing 10% FBS and 1% (100 U/ml) antibiotic-antimycotic in the incubator at 37 °C, 5% CO2 and 95% humidity for 4-5 days. (Figure 2)
- Check the confluency of OECs under an inverted microscope. Subculture the cells when the cell density reaches 80% confluence 3-4 weeks after seeding.
- Immunocytochemistry
- Seed 5 x 103 cells into each well of a 24-well culture dish.
- Wash each well three times with phosphate-buffered saline (PBS) after 24 h at room temperature.
- Fix cells with 4% paraformaldehyde in PBS for 20 min.
- Wash cells three times in PBS at room temperature for 5 min each.
- Permeate cell membranes with 0.2% Triton X-100 for 15 min.
- Wash cells three times in PBS at room temperature for 5 min each.
- Block cells with 10% bovine serum albumin for 1 h.
- Dilute the primary antibody of rabbit anti-S100-beta 1:100 in 1% BSA in PBS.
- Wash cells three times in PBS at room temperature for 5 min each.
- Dilute the secondary antibody of FITC-conjugated goat anti-rabbit 1:500 in 1% BSA in PBS.
- Incubate cells with secondary antibody of FITC-conjugated goat anti-rabbit for 2 h at room temperature in the dark.
- Wash cells three times in PBS at room temperature for 5 min each.
- Image cells by an inverted fluorescence microscopy (Figure 3).
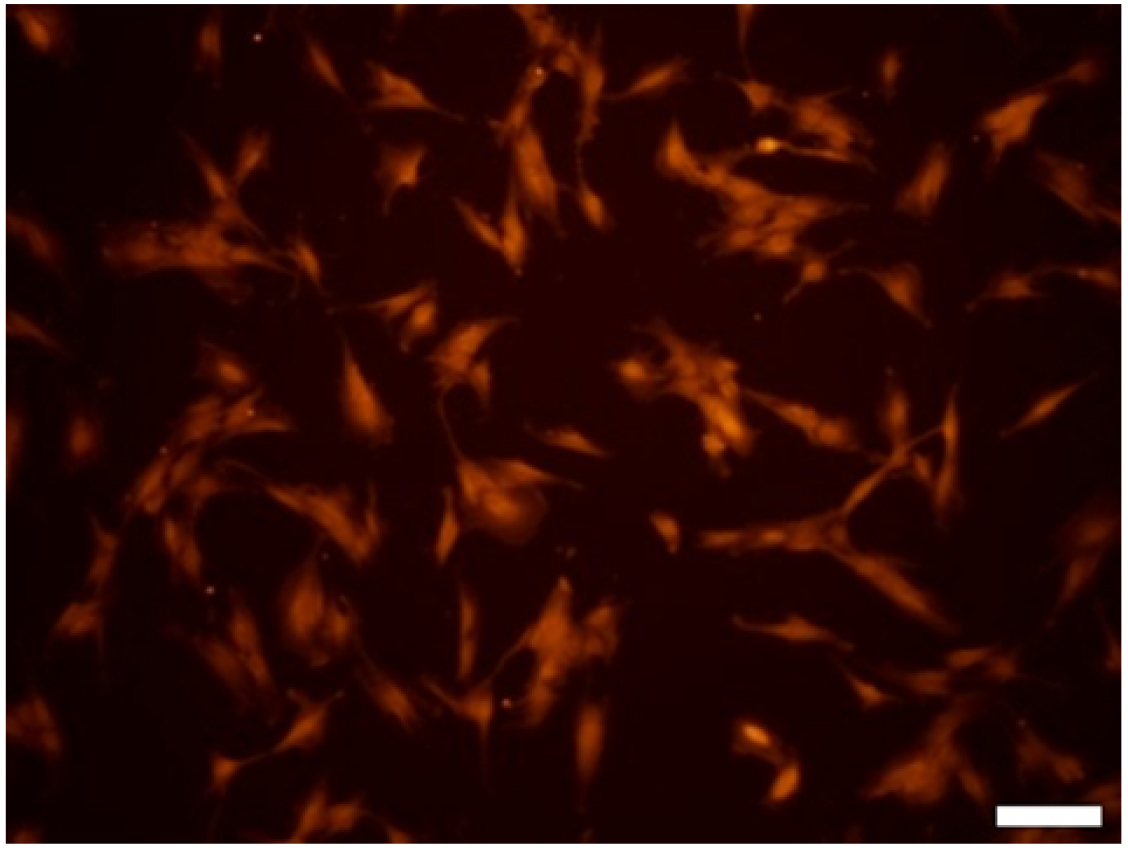
Figure 3. Purified primary olfactory ensheathing cell culture. OECs have expressed antigen of S100-beta. Scale bar = 50 µm.
- Seed 5 x 103 cells into each well of a 24-well culture dish.
Data analysis
Confirmation of cultured primary OECs was done by detecting S100-beta antigen via fluorescence immunocytochemical analysis. This analysis demonstrated that cells expressed S100-beta antigen. Test results are obtained from three independent experiments.
Notes
- Most steps should be performed in a sterile ventilation hood.
- All reagents should be pre-warmed at 37 °C before use. Exception is for washing olfactory mucosa tissue with cold PBS.
- If fibroblast contamination observed in the primary culture, cytosine arabinoside should be added to a final concentration of 5 µg/ml in the cell culture medium in the next 10 days. Cytosine arabinoside is a growth inhibitor of fibroblast.
Recipes
- Culture medium (200 ml,10% FBS, 1% antibiotic-antimycotic)
178 ml DMEM/F12
20 ml FBS
2 ml antibiotic-antimycotic
Store at 4 °C - Phosphate buffer saline (PBS) (500 ml)
1 PBS tablet dissolved in 500 ml MiliQ water
Filter and store at 4 °C - 4% paraformaldehyde
4 g paraformaldehyde dissolved in 100 ml PBS - 0.2% Triton X-100
10 µl Triton X-100 dissolved in 5 ml PBS - 10% bovine serum albumin
Dissolve 0.5 g bovine serum albumin in 5 ml PBS
Acknowledgments
This study was supported by grant number 20035 obtained from School of Advanced Technologies in Medicine and grant 19925 from Brain and Spinal Cord injury Research Center, Tehran University of Medical Sciences. The protocol was adapted from Hashemi et al. (2016).
References
- Escada, P. A., Lima, C. and da Silva, J. M. (2009). The human olfactory mucosa. Eur Arch Otorhinolaryngol 266(11): 1675-1680.
- Hashemi, M., Fallah, A., Aghayan, H. R., Arjmand, B., Yazdani, N., Verdi, J., Ghodsi, S. M., Miri, S. M. and Hadjighassem, M.R. (2016). A new approach in gene therapy of glioblastoma multiforme: human olfactory ensheathing cells as a novel carrier for suicide gene delivery. Mol Neurobiol 53(8): 5118-5128.
- Huang, Z. H., Wang, Y., Cao, L., Su, Z. D., Zhu, Y. L., Chen, Y. Z., Yuan, X. B. and He, C. (2008). Migratory properties of cultured olfactory ensheathing cells by single-cell migration assay. Cell Res 18(4): 479-490.
- Novikova, L. N., Lobov, S., Wiberg, M. and Novikov, L. N. (2011). Efficacy of olfactory ensheathing cells to support regeneration after spinal cord injury is influenced by method of culture preparation. Exp Neurol 229(1): 132-142.
- Pastrana, E., Moreno-Flores, M. T., Avila, J., Wandosell, F., Minichiello, L. and Diaz-Nido, J. (2007). BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochem Int 50(3): 491-498.
- Singh, N., Gopal, S. C., Srivastava, R. N., Chandra, T., Agarwal, S. P., Singh, S. K., Gupta, D. K. and Balapure, A. K. (2013). In vitro maintenance of olfactory mucosa: with enriched olfactory ensheathing cells. J Stem Cell Res Ther 3:1-8.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hashemi, M. and Hadjighassem, M. (2017). Primary Olfactory Ensheathing Cell Culture from Human Olfactory Mucosa Specimen. Bio-protocol 7(10): e2275. DOI: 10.21769/BioProtoc.2275.
Category
Neuroscience > Sensory and motor systems > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link