- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Analysis of Cancer Stromal Reaction Using an O-ring Co-culture Assay
Published: Vol 7, Iss 4, Feb 20, 2017 DOI: 10.21769/BioProtoc.2131 Views: 9332
Reviewed by: Guillermo GomezAndrea IntroiniJingli Cao

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assessing Human Treg Suppression at Single-Cell Resolution Using Mass Cytometry
Jonas Nørskov Søndergaard [...] James B. Wing
Aug 20, 2025 2912 Views

Isolation and Co-culture of Paneth Cells and Intestinal Stem Cells
Ryosuke Isotani [...] Toshimasa Yamauchi
Sep 20, 2025 3462 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 222 Views
Abstract
We have developed a 2D heterotypic co-culture technique between fibroblasts and cancer cells that enables the study of the stromal reaction. For such, stromal cells are seeded and cultured immediately around a tumour cell line, and the cells establish cell-cell contacts, as well as a gradient of soluble factors throughout the stromal cells, similar to that found in tissues. Thus, this system also enables the researcher to distinguish between events that are caused by direct cell-cell contact and secreted factors.
Keywords: CancerBackground
The growth and survival of a tumour within a tissue depends upon interactions with surrounding stromal cells, such as fibroblasts, inflammatory cells, endothelial cells and lymphatic cells. Research has shown that as tumours grow there is extensive cross-talk between the cancer cells and the surrounding fibroblasts. Moreover, the tumour cells may activate these fibroblasts into tumour-associated fibroblasts (TAFs). In some instances, these fibroblasts may restrict tumour growth (Coulson-Thomas et al., 2011 and 2013); however, in many cases these TAFs aid tumour cell growth and survival (Coulson-Thomas et al., 2010 and 2015). Therefore, in vitro cancer studies should also take into account the protective effects TAFs can have on cancer cells. Taking this into account, we developed a 2D heterotypic co-culture technique between fibroblasts and cancer cells that enables the study of TAFs and cancer cells in the same system.
Materials and Reagents
- 24-well clear flat bottom TC-treated Multiwell cell culture plate (Corning, Falcon®, catalog number: 353047 )
- Circular glass coverslips, FisherbrandTM cover glasses, diameter (metric) 12 mm (Thermo Fisher Scientific, Fisher Scientific, catalog number: 12-545-80 )
- O-Rings, PYREX® cloning cylinder (10 x 10 mm) (Corning, catalog number: 3166-10 )
- Stromal cells, prostate fibroblasts (WPMY-1) (ATCC, catalog number: CRL-2854TM )
- Prostate cancer PC-3 cells (ATCC, catalog number: CRL-1435TM )
- Trypsin/EDTA 0.25% (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056 )
- Phosphate buffer saline (PBS) pH 7.4, without calcium, magnesium and phenol red
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM)
- Primary antibodies
- Anti-collagen I (Calbiochem I-8H5, San Diego, CA and Sigma COL1, Sigma-Aldrich, catalog number: C2456 )
- Anti-collagen IV, against α1 type IV (Santa Cruz Biotechnology, catalog number: sc-29010 )
- Anti-collagen V (EMD Millipore, catalog number: AB763P and Abcam, catalog number: ab134800 )
- Rabbit anti-fibromodulin H-50 (Santa Cruz Biotechnology, catalog number: sc-33772 )
- Rabbit anti-biglycan H-150 (Santa Cruz Biotechnology, catalog number: sc-33788 )
- Mouse anti-fibronectin (BD, BD Transduction LaboratoriesTM, catalog number: 610077 )
- Goat anti-perlecan L-20 (Santa Cruz Biotechnology, catalog number: sc-27449 )
- Anti-versican H-56 (Santa Cruz Biotechnology, catalog number: sc-25831 )
- Mouse anti-smooth muscle α actin conjugated with Cy3 (Sigma-Aldrich, catalog number: clone 1A4 )
- Alexa Fluor® 488 or Alexa Fluor® 594 (Thermo Fisher Scientific, Molecular Probes/Invitrogen, Eugene, OR)
- Dulbecco’s modified Eagle medium (DMEM) culture medium (Thermo Fisher Scientific, GibcoTM, catalog number: 11965092 )
- L-glutamine-penicillin-streptomycin (100x) (Thermo Fisher Scientific, GibcoTM, catalog number: 10378016 )
- Paraformaldehyde (aqueous solution: 16%) (Electron Microscopy Sciences, catalog number: 15700 )
- Complete culture medium (see Recipes)
- 4% paraformaldehyde (see Recipes)
Equipment
- Ultra-Fine forceps with a straight tip (Sterilized) (Fine Science Tools, catalog number: 11399-80 )
- CO2 cell culture incubator (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 51026280 )
- Table top centrifuge (Eppendorf, model: 5702RH )
- Automatic cell counter or hemocytometer
- Biological safety cabinets (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 51026639 )
Note: The cell culture experiments must be carried out in sterile conditions. - Inverted fluorescence microscope (Zeiss, model: Observer.Z1 with Apotome )
Software
- Prism-GraphPad software
Procedure
- Preparing the O-ring co-culture system
- The fibroblasts and prostate cancer cells should be maintained as separate cell lines, according to the supplier’s guidelines.
Note: We maintained PC3 cells and fibroblasts in DMEM complete medium. The cells were passaged at 80-90% confluence and centrifuged at 300 x g. - For the heterotypic co-culture system the fibroblasts and prostate cancer cells are removed from the culture dishes with trypsin/EDTA and centrifuged.
- The cell pellets are suspended in complete culture medium.
- A sterile O-ring is placed on top of glass coverslips in 24-well polystyrene culture dishes.
Note: No silicone should be used to secure the O-ring to the coverslip since it would after affect the migration of the cells over the cell-free area. The O-ring simply sits on the cover slip; therefore care should be taken while transporting the microplate into the incubator. - Fibroblasts (1.5 x 104 in 350 μl) are placed around the O-ring and prostate cancer cells (0.5 x 104 in 100 μl) are placed inside the O-ring, as represented in Figure 1.
Note: Steps A1 to A7 must be carried out in a biosafety cabinet to ensure culture sterility and operator’s safety.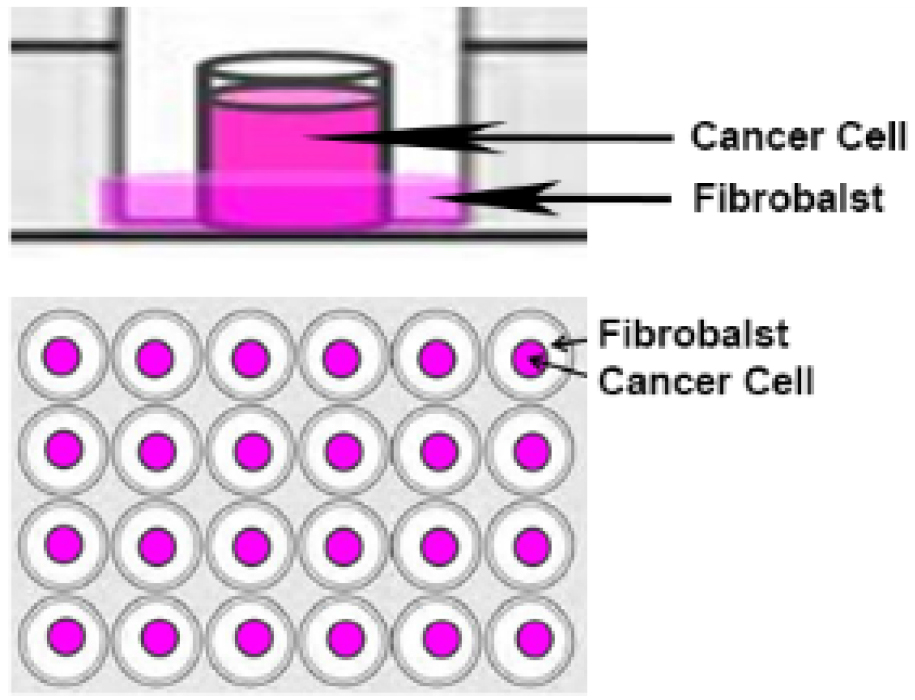
Figure 1. O-ring co-culture technique. Fibroblasts are seeded around the O-ring and cancer cells are seeded inside the O-ring. - The cells are maintained in culture with the O-rings for 24 h at 37 °C and 5% CO2.
- The O-rings are then removed with a forceps and the cells are washed twice with complete medium to remove any cells that have not adhered, and then left in culture for a further 48 h at 37 °C and 5% CO2.
Note: Upon the removal of the O-ring the investigator can ascertain the O-ring area is cell-free, which is an indication that there was no cross-contamination between cell lines. Over the next 24 h the two cell lines readily migrate into the cell-free area forming a zone containing both cell lines in direct contact. - For immunocytochemistry the cells are fixed in 4% paraformaldehyde prepared in PBS after 24 or 48 h.
Note: The investigators should monitor their cells under a microscope in order to determine the optimal time for fixing their specific cell types. We determined 24 h was optimal for most cells types.
- The fibroblasts and prostate cancer cells should be maintained as separate cell lines, according to the supplier’s guidelines.
- Immunofluorescence staining of the ECM molecules
- The cells are washed with PBS and incubated in blocking solution (5% FBS) for 1 h at room temperature.
- The cells are then incubated with primary antibodies overnight at 4 °C. The primary antibodies used in our published work were: anti-collagen I (Calbiochem I-8H5 and Sigma COL1), anti-collagen IV (against α1 type IV) and two distinct anti-collagen V (Chemicon AB763P and Abcam Clone V-3 C9), rabbit anti-fibromodulin (Santa Cruz H-50), rabbit anti-biglycan (Santa Cruz H-150), mouse anti-fibronectin, goat anti-perlecan (Santa Cruz L-20), anti-versican (Santa Cruz H-56) and finally, mouse anti-smooth muscle α actin conjugated with Cy3 (clone 1A4).
- The coverslips are then washed three times for 15 min and incubated for 1 h at room temperature with appropriate fluorescent secondary antibodies conjugated to Alexa Fluor® 488 or Alexa Fluor® 594.
- The coverslips are then washed with PBS three times, 15 min for each wash and mounted on glass slides in Fluoromount G (2:1 in PBS).
- Negative controls must be done for immunostaining experiments. These were done in our set of experiments by omitting the primary antibody. Coverslips are examined using scanning confocal inverted microscope (Zeiss LSM510) and fluorescence quantified using a LSM image browser.
- The cells are washed with PBS and incubated in blocking solution (5% FBS) for 1 h at room temperature.
Data analysis
This method has previously been used to analyse the prostate and colorectal stromal reaction (Coulson-Thomas et al., 2010 and 2011). In these studies the extracellular matrix (ECM) production was analysed in the fibroblast only region, cancer cell only region and interface which contained both the fibroblasts and cancer cells. The interface containing both fibroblasts and cancer cells can be viewed in Figure 2. The ECM was analysed by immunocytochemistry using a range of antibodies against different ECM components. We repeated the experiments at least three times in triplicates which yielded statistically significant results, with the Student’s t-test using the Prism-GraphPad software.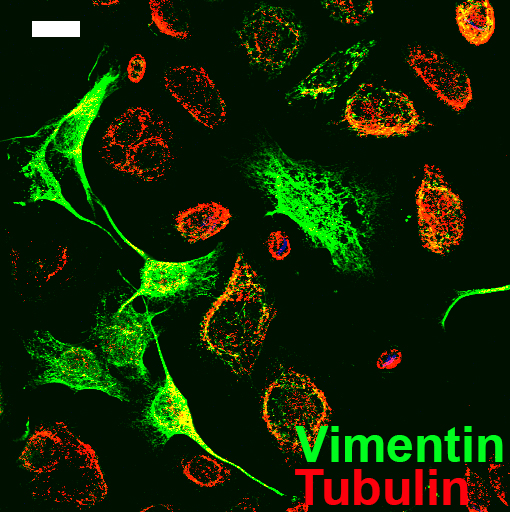
Figure 2. Image of the O-ring area containing both the prostate cancer cells and fibroblasts. Fibroblasts are evidenced by vimentin staining in green and the prostate cancer cells are evidenced by tubulin staining in red. Scale bar = 20 μm.
Notes
- This protocol was developed to study the inter-play between stromal cells and tumour cells; however, it could be used with any two cell types.
- This co-culture technique is an interesting platform for testing the effect of anti-tumour drugs on tumour cells, activated fibroblasts and tumour cells within the activated fibroblast microenvironment, which is closer to the physiological environment.
- The cell numbers seeded within the O-ring and around the O-ring should be adapted when using different cell lines according to their proliferation.
- The O-rings and coverslips were autoclaved prior to use.
Recipes
- Complete culture medium
DMEM
Fetal bovine serum (FBS) (final concentration: 10%)
L-glutamine-penicillin-streptomycin (1x) - 4% paraformaldehyde
Paraformaldehyde (final concentration: 4%)
PBS
Acknowledgments
This protocol was from Coulson-Thomas et al. (2010). This work was funded by FAPESP, CAPES and CNPq.
References
- Coulson-Thomas, V. J., Coulson-Thomas, Y. M., Gesteira, T. F., de Paula, C. A., Mader, A. M., Waisberg, J., Pinhal, M. A., Friedl, A., Toma, L. and Nader, H. B. (2011). Colorectal cancer desmoplastic reaction up-regulates collagen synthesis and restricts cancer cell invasion. Cell Tissue Res 346(2): 223-236.
- Coulson-Thomas, V. J., Coulson-Thomas, Y. M., Gesteira, T. F., Andrade de Paula, C. A., Carneiro, C. R., Ortiz, V., Toma, L., Kao, W. W. and Nader, H. B. (2013). Lumican expression, localization and antitumor activity in prostate cancer. Exp Cell Res 319(7): 967-981.
- Coulson-Thomas, V. J., Gesteira, T. F., Coulson-Thomas, Y. M., Vicente, C. M., Tersariol, I. L., Nader, H. B. and Toma, L. (2010). Fibroblast and prostate tumor cell cross-talk: fibroblast differentiation, TGF-β, and extracellular matrix down-regulation. Exp Cell Res 316(19): 3207-3226.
- Coulson-Thomas, Y. M., Gesteira, T. F., Norton, A. L., Kao, W. W., Nader, H. B. and Coulson-Thomas, V. J. (2015). The role of proteoglycans in the reactive stroma on tumor growth and progression. Histol Histopathol 30(1): 33-41.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Coulson-Thomas, V. J. (2017). Analysis of Cancer Stromal Reaction Using an O-ring Co-culture Assay. Bio-protocol 7(4): e2131. DOI: 10.21769/BioProtoc.2131.
Category
Cancer Biology > Invasion & metastasis > Tumor microenvironment
Cell Biology > Cell isolation and culture > Cell differentiation
Cell Biology > Cell isolation and culture > Co-culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









