- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assay to Evaluate BAL Fluid Regulation of Fibroblast α-SMA Expression
Published: Vol 6, Iss 22, Nov 20, 2016 DOI: 10.21769/BioProtoc.2009 Views: 8979
Reviewed by: Ivan ZanoniAchille BroggiBenoit Stijlemans

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
Keyu Guo [...] Shuyi Si
Jul 20, 2025 2277 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3547 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 482 Views
Abstract
Because transforming growth factor-β (TGF-β1) induces differentiation of fibroblasts to myofibroblasts, we developed a protocol to evaluate alveolar macrophage-derived TGF-β1 regulation of lung fibroblast differentiation (Larson-Casey et al., 2016). The protocol evaluates the ability of mouse bronchoalveolar lavage (BAL) fluid to alter fibroblast differentiation. Fibroblast differentiation was measured by the expression of α-smooth muscle actin (α-SMA).
Background
Alveolar macrophages play an integral role in pulmonary fibrosis development by increasing the expression of TGF-β1 (He et al., 2011). Our prior data demonstrate that alveolar macrophages are a critical source of TGF-β1 as mice harboring a conditional deletion of TGF-β1 in macrophages were protected from pulmonary fibrosis (Larson-Casey et al., 2016). The expression of α-SMA is a defining feature of myofibroblasts, and TGF-β1 is a well-characterized pro-fibrotic mediator that induces transformation of fibroblasts to myofibroblasts both in vitro (Desmoulière et al., 1993) and in vivo (Sime et al., 1997). Prior studies exposed fibroblasts to recombinant TGF-β1 to show its effect on differentiation and function (Horowitz et al., 2007). Here we have developed a protocol for determining the ability of mouse BAL fluid to alter the differentiation of human lung fibroblasts to myofibroblasts, the cells that produce extracellular matrix proteins.
Materials and Reagents
- 6-well cell culture plates (Corning, Costar®, catalog number: 3516 )
- Normal human fibroblasts (IMR-90) (ATCC, catalog number: CCL-186 )
- DMEM
- Fetal bovine serum (FBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 26140095 )
- Penicillin-streptomycin (10,000 U/ml, 10,000 µg/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- Amphotericin B (Thermo Fisher Scientific, GibcoTM, catalog number: 15290018 )
- RPMI 1640 medium, no phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 11835030 )
- DPBS (Thermo Fisher Scientific, GibcoTM, catalog number: 14190144 )
- NP-40
- Sodium chloride (NaCl)
- Protease inhibitor tablets (Sigma-Aldrich, catalog number: 11836170001 )
- Phosphatase inhibitor (EMD Millipore, catalog number: 524625 )
- α-SMA antibody (American Research Products, catalog number: 03-61001 )
- β-actin antibody (Sigma-Aldrich, catalog number: A5441 )
- Tween 20
- Fibroblast culture media (see Recipes)
- Lysis buffer (see Recipes)
Equipment
- Cell culture incubator, 37 °C, 5% CO2: 95% air atmosphere (Thermo Fisher Scientific, FormaTM, model: Direct Heat CO2 Incubator )
Procedure
- Seed fibroblasts at a density of 1 x 106 per well of a 6-well cell culture plate in a total volume of 1 ml. Allow cells to adhere to cell culture plate (2-6 h).
- Harvest bronchoalveolar lavage (BAL) fluid from mice (Han and Ziegler, 2013). Mice can be treated with bleomycin (1.75 U/kg) or saline as a negative control. Determine the protein concentration of bronchoalveolar lavage (BAL) fluid. Using RPMI 1640 media, normalize all BAL samples to the same protein concentration in a total volume of 1 ml.
Notes: - Typically, 0.5-1 ml of BAL fluid is used and RPMI is used to normalize the protein concentration to a final total volume of 1 ml.
- Alternatively, this assay can be performed in vitro using conditioned media from macrophage cell lines or bone marrow derived macrophages. Harvest the conditioned media from macrophages post-transfection or post-treatment, using 1 million cells per 1 ml of media.
- Remove media from fibroblasts and rinse with 1.5 ml room temperature 1x PBS, add normalized BAL fluid to fibroblasts.
- Incubate fibroblasts with BAL fluid for 24 h in a cell culture incubator, 37 °C, 5% CO2: 95% air atmosphere.
- Harvest fibroblasts by lysing cells in lysis buffer (see Recipes).
- Determine α-SMA expression by immunoblot analysis. The expression of α-SMA protein was determined by Western blotting using 20 μg of total cellular protein. After blocking in 5% milk, the membranes were probed with mouse α-SMA primary antibody using a 1:3,000 dilution in Tris buffered saline containing 0.1% Tween 20, the membranes were incubated with the anti-mouse secondary antibody (1:2,000) in antibody dilution buffer for 1 h.
Data analysis
Results can be shown as a representative immunoblot (Figure 1); also see Larson-Casey et al. (2016).
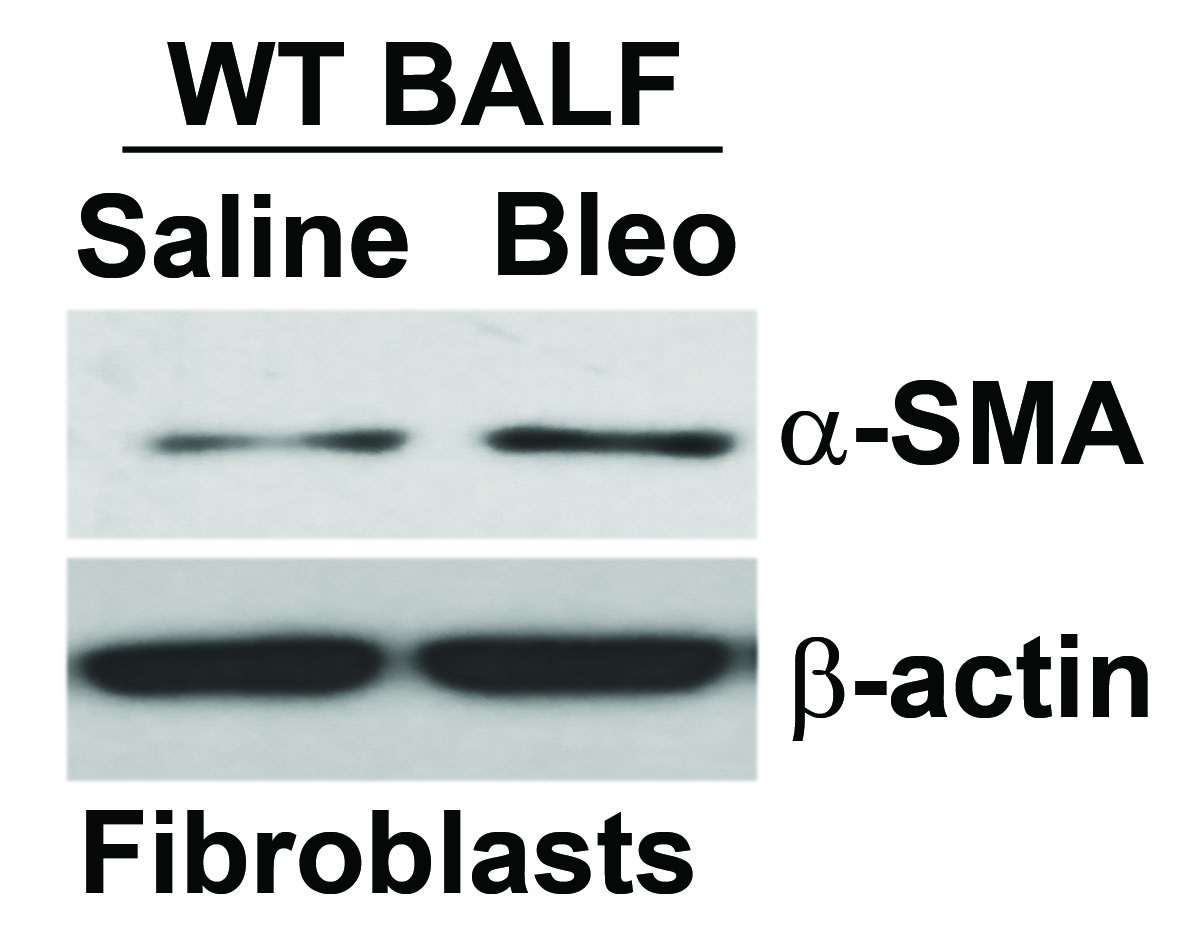
Figure 1. Immunoblot analysis of α-SMA in IMR-90 fibroblasts cultured in BAL fluid (BALF) from saline or bleomycin (Bleo) exposed WT mice
Recipes
- Fibroblast culture media
DMEM
10% heat-inactivated FBS
100 U/ml, 100 μg/ml penicillin-streptomycin
1.25 μg/ml amphotericin B (fungizone)
- Lysis buffer
1% NP-40
0.15 M NaCl
0.05 M Tris pH 7.4
1 protease tablet
Phosphatase inhibitor diluted 1:100
Acknowledgments
This work was supported by 2R01ES015981 & VA merit review BX001135.
References
- Desmoulière, A., Geinoz, A., Gabbiani, F. and Gabbiani, G. (1993). Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122(1): 103-111.
- Han, H. and Ziegler, S. F. (2013). Bronchoalveolar lavage and lung tissue digestion. Bio-protocol 3(16): e859.
- He, C., Murthy, S., McCormick, M. L., Spitz, D. R., Ryan, A. J. and Carter, A. B. (2011). Mitochondrial Cu,Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J Biol Chem 286(17): 15597-15607.
- Horowitz, J. C., Rogers, D. S., Sharma, V., Vittal, R., White, E. S., Cui, Z. and Thannickal, V. J. (2007). Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal 19(4): 761-771.
- Larson-Casey, J. L., Deshane, J. S., Ryan, A. J., Thannickal, V. J. and Carter, A. B. (2016). Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44(3): 582-596.
- Sime, P. J., Xing, Z., Graham, F. L., Csaky, K. G. and Gauldie, J. (1997). Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 100(4): 768-776.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Larson-Casey, J. L. and Carter, A. B. (2016). Assay to Evaluate BAL Fluid Regulation of Fibroblast α-SMA Expression. Bio-protocol 6(22): e2009. DOI: 10.21769/BioProtoc.2009.
Category
Immunology > Immune cell function > Macrophage
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










