- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Reconstruction of the Mouse Inflammasome System in HEK293T Cells
Published: Vol 6, Iss 21, Nov 5, 2016 DOI: 10.21769/BioProtoc.1986 Views: 11885
Reviewed by: Ivan ZanoniMeenal SinhaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In vivo Electroporation of Skeletal Muscle Fibers in Mice
Steven J. Foltz [...] Hyojung J. Choo
Jul 5, 2023 1823 Views

Primary Neuronal Culture and Transient Transfection
Shun-Cheng Tseng [...] Eric Hwang
Jan 20, 2025 2702 Views

Proliferation Assay Using Cryopreserved Porcine Peripheral Mononuclear Cells Stimulated With Concanavalin A and Analyzed With FCS ExpressTM 7.18 Software
Marlene Bravo-Parra [...] Luis G. Giménez-Lirola
Jun 5, 2025 2618 Views
Abstract
The NLRP3 (NLR family, Pyrin domain containing 3) inflammasome is a multiprotein complex comprised of NLRP3, pro-caspase-1, the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), and the protein kinase NIMA related kinase 7 (NEK7) (Shi et al., 2016; He et al., 2016; Schmid-Burgk et al., 2016). When cells are exposed to microbes and/or danger signals, the inflammasome assembles and serves as a platform for the activation of caspase-1. Caspase-1 activation promotes the processing and secretion of the pro-inflammatory cytokines interleukin-1β (IL-1β), IL-18, and IL-33 as well as pyroptosis induction (Gross et al., 2011; Arend et al., 2008), which elicit inflammatory responses. Here, we describe how to co-transfect the NLRP3 inflammasome components into HEK293T cells, which enables inflammasome activation and the production of IL-1β upon stimulation with nigericin.
Background
Inflammasomes are multiprotein complexes that control inflammatory responses and coordinate immune responses against invading microbes. Reconstitution of the NLRP3 inflammasome in vitro provides an easy and efficient way to study the regulation of inflammasome activation. In this protocol, NEK7 was introduced into the in vitro NLRP3 inflammasome system and the ratio among the NLRP3 inflammasome components was optimized, making the reconstituted NLRP3 inflammasome more similar to the physiological inflammasome in vivo. Nigericin was used to activate the inflammasome as we have observed that it induces a rapid rate of IL-1β secretion compared to other inflammasome activators. Using this protocol, the levels of IL-1β can be assayed to determine NLRP3 inflammasome function under physiological conditions as well as after gene knockdown or overexpression.
Materials and Reagents
- Costar 24 well clear TC-treated multiple well plate (Corning, Costar®, catalog number: 3527 )
- 1.5 ml Eppendorf tubes
- HEK293T cells (ATCC, catalog number: CRL-11268 )
- Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher Scientific, GibcoTM, catalog number: 10569-044 )
- Fetal bovine serum (FBS) (Gemini Bio-Products, catalog number: 100-106 )
- Penicillin-streptomycin (10,000 U/ml; 10,000 μg/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-163 )
- Pro-IL-1β-Flag, NLRP3-Flag, ASC1-Flag, pro-caspase-1-Flag and NEK7-HA plasmids
Note: Pro-IL-1β, NLRP3, ASC1, pro-caspase-1, and NEK7 were amplified by standard PCR techniques and were subsequently inserted into mammalian expression vectors using the In-Fusion® HD cloning kit per manufacturer’s instructions (click here for a detailed protocol and Shi et al., 2016). All plasmids were submitted to Addgene.
pCMV-pro-IL1β-C-Flag (Addgene, catalog number: 75131 )
pcDNA3-N-Flag-NLRP3 (Addgene, catalog number: 75127 )
pcDNA3-N-Flag-ASC1 (Addgene, catalog number: 75134 )
pcDNA3-N-Flag-Caspase-1 (Addgene, catalog number: 75128 )
pcDNA3-N-HA-NEK7 (Addgene, catalog number: 75142 ) - IL-1β ELISA Kit (affymetrix ,eBioscience, catalog number: 88-7013-76 )
- Plasmid Plus Midi Kit (QIAGEN, catalog number: 12943 )
- LB medium (MP Bio, catalog number: 3002-31 )
- Opti-MEM® (Thermo Fisher Scientific, GibcoTM, catalog number: 51985-034 )
- Lipofectamine® 2000 transfection reagent (Thermo Fisher Scientific, InvitrogenTM, catalog number: 11668-019 )
- Nigericin (Sigma-Aldrich, catalog number: N7143-10MG )
- Ethanol
- SuperSignalTM West Pico chemiluminescent substrate (Thermo Fisher Scientific)
- DMEM cell culture medium (see Recipes)
- Nigericin stock solution (see Recipes)
Equipment
- 37 °C, 5% CO2 forced-air incubator (Thermo Fisher Scientific, Fisher Scientific, model: Sanyo Incubator Panasonic MCO-19AIC(UV) CO2 )
- Shaker incubator (Eppendorf, model: New Brunswick I24 )
- Pipettes (Mettler-Toledo, ShopRainin)
- Microcentrifuge (Eppendorf, model: Centrifuge 5424 )
- ELISA plate reader (Bio Tek Instruments, model: Synergy Neo2 Multi-Mode Reader )
- Spectrophotometer (Thermo Fisher Scientific, Thermo ScientificTM, model: NanoDrop 2000c Spectrophotometer )
Software
- GraphPad Prism 6 software (http://www.graphpad.com/scientific-software/prism/)
Procedure
- Plate HEK293T cells in 24-well microplates at a density of 2 x 105 cells per well in 0.5 ml complete DMEM cell culture medium and incubate at 37 °C in a forced-air incubator overnight.
- Extract the pro-IL-1β-Flag, NLRP3, ASC, pro-caspase-1 and NEK7 plasmids from their respective bacterial cultures (35 ml) by using a QIAGEN Plasmid Plus Midi Kit according to the manufacturer’s instructions.
Note: Click here for a detailed protocol on how to prepare a liquid culture of the Addgene plasmid. Briefly, add 35 ml of LB medium (with the appropriate antibiotic) to a tube or flask for each Addgene plasmid. Inoculate the LB medium using a sterile pipette tip and incubate 12-18 h at 37 °C in a shaking incubator. - In 1.5 ml Eppendorf tubes, dilute 1.5 μl of Lipofectamine® 2000 in 100 μl Opti-MEM per well.
- In 1.5 ml Eppendorf tubes, dilute the pro-IL-1β-Flag (200 ng/well), ASC (20 ng/well), pro-caspase-1 (100 ng/well), NLRP3 (200 ng/well) and NEK7 (200 ng/well) plasmids in 100 μl Opti-MEM.
Note: Avoid transfecting more than the amount indicated for the ASC plasmid. Excess ASC promotes self-aggregation and induces NLRP3-independent caspase-1 activation. - Add the diluted DNA to the diluted Lipofectamine® 2000 (1:1 ratio), incubate for 5 min at room temperature, and then add the DNA-lipid complex to the HEK293T cells in 0.5 ml DMEM cell culture medium.
- 24-36 h after transfection, replace the medium with 250 μl DMEM cell culture medium.
- Supernatants are collected 6-12 h after media change.
- Optional: To stimulate the NLRP3 inflammasome, 10 μg/ml nigericin can be directly added to the 250 μl DMEM media one hour before supernatant collection (step 7).
- ELISA (or Western blot) to assay the amount of mature IL-1β in the supernatants. Perform ELISA according to the manufacturer’s instructions.
Note: When using Western blot to detect the mature IL-1β, the supernatants do not need to be concentrated. Mature IL-1β in 15 μl of supernatant could be detected by SuperSignalTM West Pico chemiluminescent substrate (Thermo Fisher Scientific).
Data analysis
ELISA data should be collected from a minimum of two independent experiments with at least 3 replicates per treatment. Figure 1 shows representative ELISA data after transfection of the indicated plasmids into HEK293T cells. The statistical significance of the differences between samples can be determined with GraphPad Prism 6 software and Student’s t-test (unpaired, two-tailed). A P value of < 0.05 is considered statistically significant.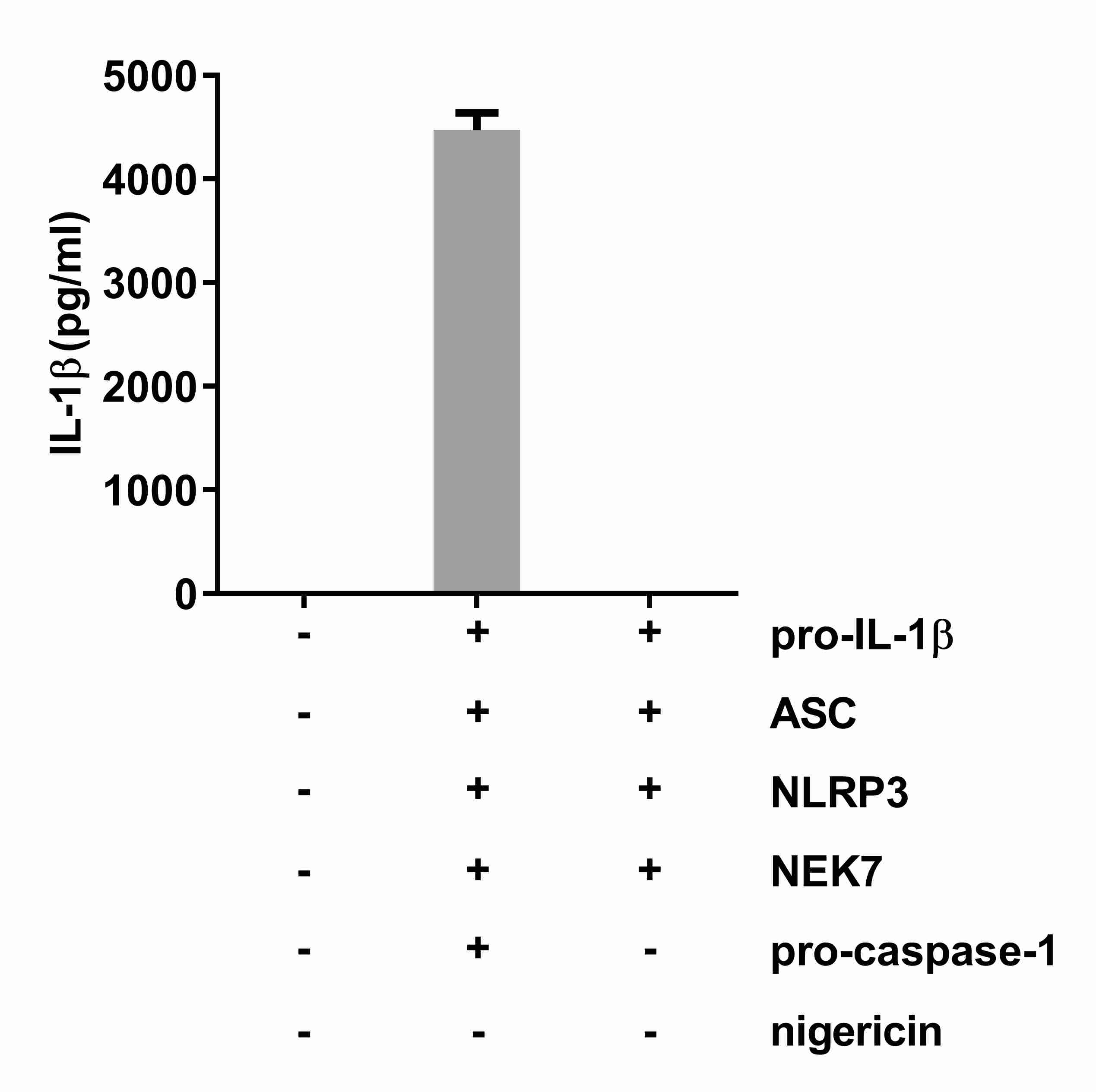
Figure 1. Levels of secreted IL-1β from reconstituted HEK293T cells. Transfected components in each sample are indicated below the X-axis. 50 μl supernatants of each HEK293T cell culture sample were used for the IL-1β ELISA. Representative data were graphed using GraphPad Prism 6 software.
Recipes
- DMEM cell culture medium
Mix 500 ml DMEM with 50 ml FBS and 5 ml penicillin-streptomycin (10,000 U/ml; 10,000 μg/ml)
Store at 4 °C until use - Nigericin stock solution
Dissolve 10 mg nigericin in 1 ml pure ethanol and mix thoroughly
Store at -80 °C until use
Acknowledgments
This protocol was adapted from the previously published study, Lu et al. (2012) and was performed by Shi et al. (2016). This work was supported by the US National Institutes of Health (U19 AI100627).
References
- Arend, W. P., Palmer, G. and Gabay, C. (2008). IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev 223: 20-38.
- Gross, O., Thomas, C. J., Guarda, G. and Tschopp, J. (2011). The inflammasome: an integrated view. Immunol Rev 243(1): 136-151.
- He, Y., Zeng, M. Y., Yang, D., Motro, B. and Núñez, G. (2016). NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530(7590): 354-357.
- Lu, B., Nakamura, T., Inouye, K., Li, J., Tang, Y., Lundback, P., Valdes-Ferrer, S. I., Olofsson, P. S., Kalb, T., Roth, J., Zou, Y., Erlandsson-Harris, H., Yang, H., Ting, J. P., Wang, H., Andersson, U., Antoine, D. J., Chavan, S. S., Hotamisligil, G. S. and Tracey, K. J. (2012). Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488(7413): 670-674.
- Schmid-Burgk, J. L., Chauhan, D., Schmidt, T., Ebert, T. S., Reinhardt, J., Endl, E. and Hornung, V. (2016). A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J Biol Chem 291(1): 103-109.
- Shi, H., Wang, Y., Li, X., Zhan, X., Tang, M., Fina, M., Su, L., Pratt, D., Bu, C. H., Hildebrand, S., Lyon, S., Scott, L., Quan, J., Sun, Q., Russell, J., Arnett, S., Jurek, P., Chen, D., Kravchenko, V. V., Mathison, J. C., Moresco, E. M., Monson, N. L., Ulevitch, R. J. and Beutler, B. (2016). NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol 17(3): 250-258.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Shi, H., Murray, A. and Beutler, B. (2016). Reconstruction of the Mouse Inflammasome System in HEK293T Cells. Bio-protocol 6(21): e1986. DOI: 10.21769/BioProtoc.1986.
Category
Immunology > Immune cell function > General
Molecular Biology > DNA > Transfection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









