- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Nippostrongylus brasiliensis Larvae from Mouse Lungs
Published: Vol 6, Iss 4, Feb 20, 2016 DOI: 10.21769/BioProtoc.1736 Views: 12073
Reviewed by: Ivan ZanoniHongwei HanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2540 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views
Abstract
The rodent parasite Nippostrongylus brasiliensis (N. brasiliensis) models the salient features of helminth infection including skin penetration, migration from tissues to lung, maturation and egg production in the gut. As a potent activator of systemic and mucosal Th2 immune responses, Nippostrongylus brasiliensis has been extensively used to study host protective immunity and in vivo regulation of Th2 immune response. Six to eight week old C57Bl/6J, Balb/c mice or any other strains are suitable, as all are susceptible to infection. Inocula of 150-650 L3 larvae can be administered by subcutaneous injection, but for greatest consistency a dose of 550 L3 larvae is routinely used for experimental purposes. We have optimized three different protocols for the isolation of larvae from the lungs of mice infected with the L3 stage of Nippostrongylus brasiliensis. Larvae can migrate to the lung between 18-60 h post inoculation from any site in the body. The numbers of larvae appearing in the lung peaks at 48 h after inoculation and it is recommended that isolation/harvesting be performed at 48 h for greatest consistency of each harvest method:
- Isolation by thermal induced migration-allows for the recovery of viable larvae but not dead or moribund larvae.
- Isolation by digestion of the lung-allows for the recovery of both dead and viable larvae.
- Isolation by bronchoalveolar lavage (BAL) -allows for the assessment of the number of molt 4 larvae on their way to the gut.
These protocols can be used to follow the dynamics of worm migration during infection and the effect of the host immune system on worm viability and fecundity.
Part I. Isolation by migration
Materials and Reagents
- Cheesecloth cut into 8 x 8 cm squares (any fabric store)
- 8 x 8 cm plastic disposable weigh boats (Medi'Ray-Laboratory, Runlab Archives, catalog number: RL33102 )
- 50 ml centrifuge tubes (Thermo Fisher Scientific, FalconTM, catalog number: 352070 / 14-432-22 ) and corresponding racks
- Disposable Pasteur pipettes (Interab Supply, catalog number: KJ619-1 )
- 60 x 15 mm TC dish (Thermo Fisher Scientific, FalconTM, catalog number: 353002 / 08-772B )
- N. brasiliensis infected mice (Camberis et al., 2003)
- 70% Ethanol
- PBS (DPBS, no calcium, no magnesium) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190-144 )
Equipment
- Iris scissors (Medicon, catalog number: 202.20.11 )
- Forceps (Medicon, catalog number 07.55.20 )
- Water-bath set at 37 °C (Biolab, model: Grant SUB Aqua 18 ) with water set to a depth not exceeding 2 cm from the top of the 50 ml tubes
- Stereo-microscope (Olympus, model: SZX16 )
Procedure
- Prepare the Migration tube by adding 45 ml PBS to a 50 ml tube. Keep warm in 37 °C water bath until ready to use.
- Euthanize the infected mouse by cervical dislocation or any approved form of euthanasia.
- Place mouse on its back and wet with 70% ethanol.
- Expose the thoracic cavity; remove the lungs and place in a clean disposable weigh-boat.
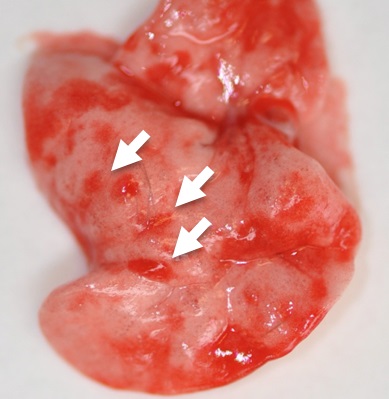
Figure 1. Day 2 lungs from N. brasiliensis infected mouse with visible petechiae (haemorraghic spots caused by the entry of larvae into the lung, indicated by the white arrows) - Dice the lungs into fine tissue pieces of approximately 1-2 mm using the scissors.
- Place an 8 x 8 cm piece of cheesecloth into a prepared 50 ml tube with the edges of the cloth hanging over the side of the vessel; this should form an open pouch in the tube (Figure 2a).
- Transfer the diced lung into the pouch of the prepared 50 ml tube (Figure 2b).
- Take the exposed edges of the cheesecloth, and close the pouch in such a way that the exposed cheesecloth edge hangs over the edge of the 50 ml tube (Figure 2c). Seal the tube with the cap so as to trap the edge of the cloth under the cap (Figure 2d).
- Place in water bath and incubate for at least 3 h at 37 °C or up to overnight in water bath.
Note: Ensure at least two-thirds of the tube is submerged in the water as the larvae migrate out of the tissue by following a thermal gradient and settle at the bottom of the tube.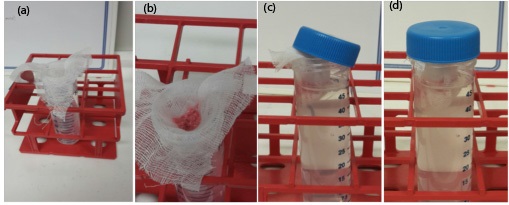
Figure 2. Tube set up for thermal gradient induced migration/isolation/enumeration of larvae from infected tissues - Discard cheesecloth following incubation period.
Note: Worms will have migrated through the tissue and collected at the bottom of the 50 ml tube. - Without disrupting the pellet, discard all but 2 to 3 ml of the PBS by aspirating with a plastic disposable pipette.
- Transfer the contents of the tube into a 60 x 15 mm TC dish and visualize the larvae using a stereo microscope.
Note: Volumes of more than 3 ml can make focusing on the larvae difficult.
Part II. Isolation by lung digest
Materials and Reagents
- 50 ml centrifuge tubes (Thermo Fisher Scientific, FalconTM, catalog number: 352070 / 14-432-22 ) and corresponding racks
- 6-well tissue culture dishes (Thermo Fisher Scientific, FalconTM, catalog number: 353046 / 08-772-1B )
- 100 μm Cell strainers (Thermo Fisher Scientific, FalconTM, catalog number: 352360 / 22-363-549 )
- 40 μm Cell strainers (Thermo Fisher Scientific, FalconTM, catalog number: 352340 / 22-363-547 )
- Disposable Pasteur pipettes (InterLab Supply, catalog number: KJ619-1 )
- N. brasiliensis infected mice (Camberis et al., 2003)
- 70% Ethanol
- PBS (DPBS, no calcium, no magnesium) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190-144 )
- LiberaseTM TL Research Grade (Sigma-Aldrich, catalog number: 5401020001 )
- DNase I (Sigma-Aldrich, catalog number: 10104159001 )
- IMDM, GlutaMAXTM Supplement (Thermo Fisher Scientific, GibcoTM, catalog number: 31980-030 )
- Digestion mix (see Recipes)
Equipment
- Iris scissors (Medicon, catalog number: 202.20.11 )
- Forceps (Medicon, catalog number: 07.55.20 )
- Stereo-microscope (Olympus, model: SZX16 )
- Incubator Shaker/Rocker (Eppendorf, New Brunswick Scientific, model: innova 4200 ) set at 37 °C
Procedure
- Euthanize the mouse by cervical dislocation or any approved form of euthanasia.
- Place mouse on its back and wet with 70% ethanol.
- Expose the thoracic cavity; remove lungs and place into a well of the 6-well dish.
- Dice the lungs into fine tissue of approximately 1-2 mm pieces using the scissors.
- Add 5 ml Digestion mix to each well and gently mix by swirling the plate.
- Incubate for 45 min at 37 °C with slow agitation in a shaking incubator.
- Using a disposable pipette, transfer the lung digest to a 100 μm cell strainer attached to a 50 ml tube. Rinse the well with 5 ml IMDM and transfer to cell strainer
- Transfer the contents of the tube to a 40 μm cell strainer attached to another 50 ml tube, using a disposable pipette.
Note: This separates the lung tissue from the larvae, the larvae staying trapped in on top of the filter. - Transfer the 40 μm strainer to a 6 well TC dish and place the 40 μm strainer upside down.
- Rinse with 5 ml IMDM using a disposable pipette to dislodge larvae trapped on the cell strainer. Inspect the cell strainer for the presence of larvae using a stereo-microscope and rinse again if necessary.
- Visualize larvae using a stereo-microscope.
Part III. Isolation by bronchoalveolar lavage (BAL)
This protocol has been adapted from protocols used in measuring allergic airway inflammation (Harris et al., 1997; Erb et al., 1998).
Materials and Reagents
- 1 ml slip lip syringes (BD, catalog number: 302100 )
- 18 G Insyte catheter (BD, catalog number: 381247 )
- 15 ml Conical Centrifuge tubes (Thermo Fisher Scientific, FalconTM, catalog number: 352096 / 14-959-49B ) and corresponding racks
- 60 x 15 mm TC dish (Thermo Fisher Scientific, FalconTM, catalog number: 353002 / 08-772B )
- N. brasiliensis infected mice (Camberis et al., 2003)
- 70% Ethanol
- PBS (DPBS, no calcium, no magnesium) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190-144 )
Equipment
- Iris scissors (Medicon, catalog number: 202.20.11 )
- Forceps (Medicon, catalog number 07.55.20 )
- Stereo-microscope (Olympus, model: SZX16 )
Procedure
- Euthanize the mouse by cervical dislocation (or any approved form of euthanasia) but taking care to maintain the integrity of the trachea.
- Place mouse on its back and wet with 70% ethanol.
- Make a midline incision, pull back the skin and expose the trachea by carefully separating the muscle tissue above the trachea (Figure 3a).
- Make a small horizontal incision across the exposed trachea without going right through the trachea (Figure 3b and Figure 3c).
- Attach an 18 G Insyte catheter to a 1 ml syringe and fill with 1 ml PBS.
- Insert the catheter approximately 10 mm into the trachea and flush the contents of the syringe into the lungs (Figure 3d).
- Draw the fluid out (Figure 3e) and place contents of syringe into a 15 ml tube.
The lavage fluid will be contaminated with blood due to the damage caused by the larvae entering the lung. To improve visualization of the larvae when lavage fluid is contaminated with blood, spin the BAL fluid down at 1,400 rpm for 2 min, remove the supernatant and lyse the red blood cells by the addition of 3ml of water to the pellet, then count larvae. Distinguishing features of the molt 4 larvae is the deposition of a dark-brown pigment that is clearly visible in the gut (Bouchery et al., 2015). - Repeat twice more with fresh PBS for each lavage.
- Visualize the larvae by transferring the lavage into a 60 x 15 mm TC dish and inspecting the contents using a stereo microscope.
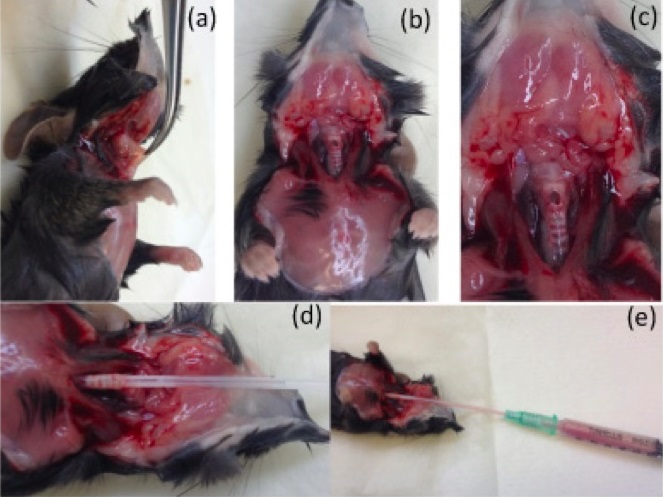
Figure 3. Experimental procedure for performing bronchoalveolar lavage
Representative data
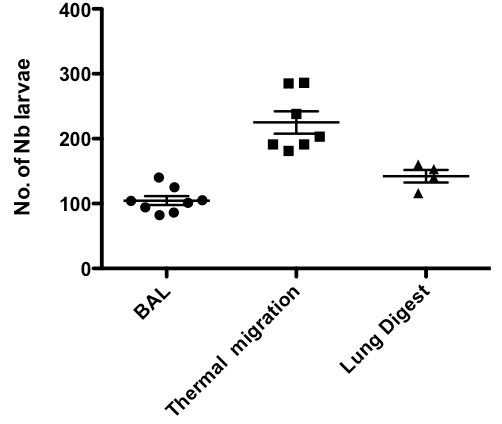
Figure 4. Comparison of larval recovery from lung using either thermal gradient induced migration, bronchoalveolar lavage (BAL) or lung digestion
Notes
For consistent results using the above protocols, it is important to always use larvae from 2-6 weeks old cultures for infecting mice. The infectivity of larvae is much reduced after 6 weeks culture (Camberis et al., 2003; Kassai, 1982).
Recipes
- Digestion mix
0.1 mg/ml Liberase TL
120 mg/ml DNase I
Made up in IMDM
Make up fresh, 5 ml per lung
Acknowledgments
This work has been adapted and modified from previous work carried out by Professor Graham Le Gros’s Allergy and Parasitic diseases laboratory, Malaghan Institute of Medical Research, New Zealand. This work was supported by The Health Research Council of New Zealand and the Marjorie Barclay Trust.
References
- Bouchery, T., Kyle, R., Camberis, M., Shepherd, A., Filbey, K., Smith, A., Harvie, M., Painter, G., Johnston, K., Ferguson, P., Jain, R., Roediger, B., Delahunt, B., Weninger, W., Forbes-Blom, E. and Le Gros, G. (2015). ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat Commun 6: 6970.
- Camberis, M., Le Gros, G. and Urban, J., Jr. (2003). Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr Protoc Immunol Chapter 19: Unit 19 12.
- Erb, K. J., Holloway, J. W., Sobeck, A., Moll, H. and Le Gros, G. (1998). Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med 187(4): 561-569.
- Harris, N., Campbell, C., Le Gros, G. and Ronchese, F. (1997). Blockade of CD28/B7 co-stimulation by mCTLA4-Hgamma1 inhibits antigen-induced lung eosinophilia but not Th2 cell development or recruitment in the lung. Eur J Immunol 27(1): 155-161.
- Harvie, M., Camberis, M., Tang, S. C., Delahunt, B., Paul, W. and Le Gros, G. (2010). The lung is an important site for priming CD4 T-cell-mediated protective immunity against gastrointestinal helminth parasites. Infect Immun 78(9): 3753-3762.
- Kassai, T. (1982). Handbook of Nippostrongylus brasiliensis, Akademia Kiado, Budapest, ISBN-10: 9630529769.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Camberis, M., Bouchery, T. and Gros, G. L. (2016). Isolation of Nippostrongylus brasiliensis Larvae from Mouse Lungs. Bio-protocol 6(4): e1736. DOI: 10.21769/BioProtoc.1736.
Category
Immunology > Animal model > Mouse
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









