- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Human Blood Component Vaccinia Virus Neutralization Assay
Published: Vol 5, Iss 23, Dec 5, 2015 DOI: 10.21769/BioProtoc.1674 Views: 9365
Reviewed by: Lee-Hwa TaiElizabeth V. ClarkeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Establishment of a Human Cell Line Persistently Infected with Sendai Virus
Christopher Coakley [...] Saurabh Chattopadhyay
Aug 20, 2017 9104 Views

A Triple-challenge Mouse Model of Allergic Airway Disease, Primary Influenza Infection, and Secondary Bacterial Infection
Sean Roberts [...] Yoichi Furuya
Apr 20, 2020 4531 Views

Environmental Conditioning and Aerosol Infection of Mice
Eriko Kudo and Akiko Iwasaki
Apr 20, 2020 5095 Views
Abstract
Many therapeutic viruses, such as oncolytic viruses, vaccines, or gene therapy vectors, may be administered by the intravenous route to maximize their delivery to target tissues. Blood components, such as antibody, complement and blood cells (such as neutrophils, monocytes, T cells, B cells or platelets) may result in viral neutralization and therefore reduce the therapeutic efficacy. This protocol will describe an in vitro assay by which to test the interaction of viruses with blood components. The effect of various factors can be isolated through fractionation. While whole blood can offer the most physiologically relevant snapshot, plasma can investigate the effects of antibody in concert with complement, and heat inactivated plasma will interrogate the effect of antibody alone.
Keywords: ComplementMaterials and Reagents
- BD Vacutainer 7 ml Glass Serum Tubes, No Additive, Silicone-Coated Interior (BD, catalog number: 366431 )
- Eppendorf tubes (1.5 ml)
- T175 cm2 culture flasks (Corning Inc., catalog number: 431079 )
- 12-well plates culture plates (Corning Inc., catalog number: 3513 )
- 15 ml Falcon tubes (BD Biosciences, Falcon®, catalog number: 352096 )
Note: Currently, it is “Thermo Fisher Scientific, FalconTM, catalog number: 352096 ”. - Insulin syringes 27G1/2” (Terumo Medical Corporation, catalog number: SS05M2713 )
- 0.22 μm Millipore® StericupTM filter unit (Merck Millipore Corporation, catalog number: SCGPU05RE )
- Alcohol wipes
- U2OS human osteosarcoma tumor cells (ATCC), grown in complete DMEM (10% FBS)
- Vaccinia virus stock ≥ 5 x 106 pfu/ml
- 1x sterile phosphate-buffered saline (PBS) (Hyclone, catalog number: 21-031-CV )
Note: Currently, it is “Mediatech, Inc., Corning, catalog number: 21-031-CV ”. - Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F1051 )
- Dulbecco’s modified Eagle medium (DMEM) culture media (Hyclone, catalog number: 10-031-CV )
Note: Currently, it is “Mediatech, Inc., Corning, catalog number: 10-031-CV ”. - 2x DMEM powder (Life Technologies, catalog number: 12800-017 )
Note: Currently, it is “Thermo Fisher Scientific, GibcoTM, catalog number: 12800-017 ”. - Carboxymethylcellulose (CMC) (Sigma-Aldrich, catalog number: C5678-1KG )
- Sodium bicarbonate (Thermo Fisher Scientific, catalog number: S233-500 )
- 100x Penicillin-Streptomycin (GE Healthcare, Hyclone, catalog number: SV30010 )
- Refludan (Lepirudin)
- Crystal violet (Sigma-Aldrich, catalog number: C0775 )
- Methanol (Thermo Fisher Scientific, catalog number: A412P-4 )
- 2x DMEM solution (see Recipes)
- 3% Carboxymethylcellulose (CMC) (see Recipes)
- Titration overlay media (see Recipes)
- Crystal violet (see Recipes)
Equipment
- Centrifuge (Thermo Fisher Scientific, model: ST40R )
- Biosafety cabinet
- 37 °C, 5% CO2 incubator (Sanyo)
- ViCell (Beckman Coulter) or hemocytometer cell counter
- pH meter
- Heat blocks set to 37 °C and 56 °C
- BD Vacutainer® Push Button Blood Collection Set with Pre-Attached Holder (example 25G x 0.75 in) (BD, catalog number: 367336 )
Procedure
- Preparation of cells for viral titration
- On the day prior to the neutralization experiment, 12 well plates should be set up with U2OS cells seeded at 2.5 x 105 cells/well. The U2OS cell line is a human osteosarcoma line that is highly infectable with Vaccinia virus and forms a very consistent monolayer, making it ideal for Vaccinia virus plaque assays. U2OS cells are cultured in DMEM with 10% FBS and should also be set up in the 12 well plates in the same media. The number of plates should correspond to the number of samples in which virus neutralization is to be assessed and account for duplicate or triplicate titrations.
- On the day prior to the neutralization experiment, 12 well plates should be set up with U2OS cells seeded at 2.5 x 105 cells/well. The U2OS cell line is a human osteosarcoma line that is highly infectable with Vaccinia virus and forms a very consistent monolayer, making it ideal for Vaccinia virus plaque assays. U2OS cells are cultured in DMEM with 10% FBS and should also be set up in the 12 well plates in the same media. The number of plates should correspond to the number of samples in which virus neutralization is to be assessed and account for duplicate or triplicate titrations.
- Collection of blood
- Clean the top of the vacutainer collection tube with an alcohol wipe.
- Venous blood should be collected by a trained phlebotomist into the vacutainer tube using a butterfly needle with connected vacutainer holder.
- Immediately after blood collection, using the insulin syringe, inject Refludan (70 μl of a 100x stock of Refludan to a final concentration of 50 μg/ml) through the rubber top of the tube. Invert several times (4-5 times) at room temperature to ensure proper mixing.
- Clean the top of the vacutainer collection tube with an alcohol wipe.
- Neutralization assay
- Prepare a 250 μl aliquot of whole blood per blood donor in an Eppendorf tube.
- Transfer remaining blood to a 15 ml falcon tube and spin at 800 x g for 5 min at room temperature to obtain plasma.
- Collect the plasma and prepare two 250 μl aliquots of plasma in Eppendorf tubes per blood donor.
- Heat inactivates one plasma sample (30 min at 56 °C) per blood donor to inactivate complement using either a heat block or a water bath.
- As a control to verify the amount of input virus under non-neutralizing conditions, prepare an additional tube with 250 μl of serum free media or PBS.
- Add 10 μl of prepared virus dilution (5 x 106 pfu/ml) to each sample and vortex very gently (final concentration is 2 x 105 pfu/ml). The total volume of each sample should be 260 μl.
- Incubate samples at 37 °C for one hour.
- Prepare a 250 μl aliquot of whole blood per blood donor in an Eppendorf tube.
- Titration of infectious virus
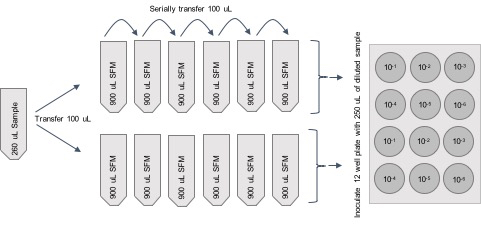
Figure 1. Titration procedure schematic- Prepare 6 Eppendorf tubes with 900 μl of SFM per sample.
- Add 100 μl of sample to 900 μl of SFM. Make 1/10 serial dilutions with the remaining five tubes. (Dilution series will be 10-1 to 10-6.)
- Transfer 250 μl of diluted sample into the wells of the 12 well plate.
- Incubate for one hour at 37 °C. Every 15-20 min, plates should be rocked to ensure that no part of the well dries out during the minimal volume infection.
- Completely remove the inoculum by aspiration and add 1 ml of prepared titration overlay media per well.
- Two days after the virus neutralization experiment, visualize the plaque assay using a 0.1% crystal violet solution. Remove overlay by aspiration and wash wells with 1x PBS. Add 0.25 ml of 0.1% crystal violet solution to wells and incubate at room temperature for 5-10 min. Remove crystal violet and collect in a container for appropriate disposal. Wash the wells with tap water and dispose of this waste with crystal violet waste. Allow wells to air dry.
- Plaques are visible by the naked eye. Enumerate the number of plaques per well and multiply by the dilution factor.
- Data can be represented as Titer (pfu/ml) or as relative recovery to the control sample. Relative recovery can be calculated by dividing the titer of the test sample by the titer of the control non-neutralized sample (where virus was spiked into PBS or DMEM).
- Prepare 6 Eppendorf tubes with 900 μl of SFM per sample.
Representative data
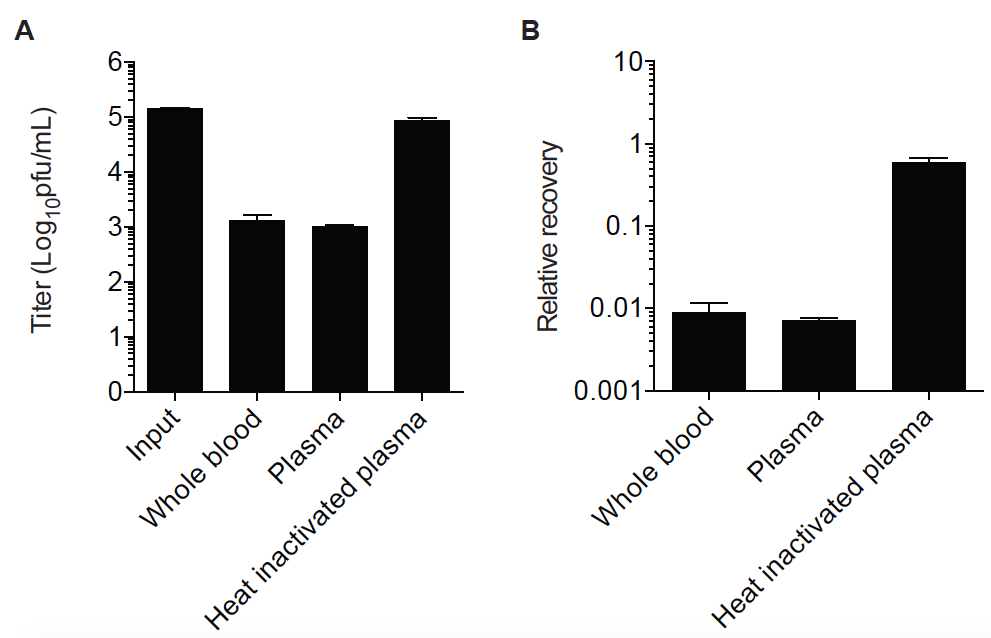
Figure 2. Infectious Vaccinia virus recovery after incubation with blood, plasma or heat inactivated plasma from one representative human donor. Data can be represented as either titer (Log10 pfu/ml) as in panel A or as a proportion of virus recovered from the input (PBS or DMEM) as in panel B.
Notes
- Human blood must be collected with informed consent from all donors and according to a protocol approved by an institutional review board. Collection of information regarding previous exposures to the viruses in question, such as vaccination, may be important for interpreting experimental results and must be done according to an approved protocol. For example, in Evgin et al. (2015) blood donors for Vaccinia virus experiments were stratified according to self-reported vaccination status and date of birth relative to the end of the smallpox vaccination campaign.
- The protocol has been described for use with Vaccinia virus, however may be easily adapted using titration methods appropriate for the virus in question.
- The choice of anti-coagulant is important for assessment of the complement cascade. EDTA, Heparin and citrate will all interfere with complement activation. Hirudin and derivatives thereof, such as Refludan, are specific thrombin inhibitors and do not interfere with complement (Bexborn et al., 2009).
- Virus neutralization can be assessed at any concentration. Similarly, the volumes of blood or plasma can be adjusted as needed. This protocol uses the concentration of 2 x 105 pfu/ml which represents the clinical dose of 1 x 109 pfu in an estimated adult human blood volume of 5 L, as described in Evgin et al. (2015).
- This protocol can be adapted to use various inhibitors or blocking antibodies. For example, prior to step C6, peptide based complement inhibitors or inhibitory monoclonal antibodies were added in Evgin et al. (2015). An additional 15 min pre-incubation at 37 °C was included to ensure binding of the peptides or antibodies prior to the addition of virus.
Recipes
- 2x DMEM (500 ml)
Dissolve 1 package of DMEM powder in 500 ml of ddH2O
Add 3.4 g of bicarbonate
Adjust pH to 7.2
Filter through a 0.22 μm Millipore® StericupTM filter unit or similar vacuum filtration system
Add 100 ml FBS before making titration overlay media
Stored at 4 °C - 3% carboxymethylcellulose (CMC) (500 ml)
Dissolve 15 g of CMC powder in 500 ml ddH2O
Autoclave
Stored at room temperature - Titration overlay media (500 ml)
250 ml of 2x DMEM (+20% FBS)
250 ml of 3% CMC
5 ml 100x penicillin/streptomycin
Stored at 4 °C - Crystal violet
Dissolve 1 g in 1 L of 80% methanol, 20% dH2O
Acknowledgments
The protocol was originally published in Evgin et al. (2015). This work was supported by the Terry Fox Research Foundation, the Ontario Institute for Cancer Research, the Ottawa Regional Cancer Foundation and the Canadian Institute for Health Research.
References
- Bexborn, F., Engberg, A. E., Sandholm, K., Mollnes, T. E., Hong, J. and Nilsson Ekdahl, K. (2009). Hirudin versus heparin for use in whole blood in vitro biocompatibility models. J Biomed Mater Res A 89(4): 951-959.
- Evgin, L., Acuna, S. A., Tanese de Souza, C., Marguerie, M., Lemay, C. G., Ilkow, C. S., Findlay, C. S., Falls, T., Parato, K. A., Hanwell, D., Goldstein, A., Lopez, R., Lafrance, S., Breitbach, C. J., Kirn, D., Atkins, H., Auer, R. C., Thurman, J. M., Stahl, G. L., Lambris, J. D., Bell, J. C. and McCart, J. A. (2015). Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol Ther 23(6): 1066-1076.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Evgin, L. and Bell, J. (2015). Human Blood Component Vaccinia Virus Neutralization Assay. Bio-protocol 5(23): e1674. DOI: 10.21769/BioProtoc.1674.
Category
Cancer Biology > Tumor immunology > Cancer therapy > Cell isolation and culture
Immunology > Complement analysis > Virus
Immunology > Host defense > Human
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










