- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cytokine-Stimulated Phosphoflow of Whole Blood Using CyTOF Mass Cytometry
Published: Vol 5, Iss 11, Jun 5, 2015 DOI: 10.21769/BioProtoc.1495 Views: 15996
Reviewed by: Yang FuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol to Isolate Germinal Centers by Laser Microdissection
Farbod Bahreini [...] Kathrin Kalies
Jun 5, 2022 2523 Views

Immunohistochemistry of Immune Cells and Cells Bound to in vivo Administered Antibodies in Liver, Lung, Pancreas, and Colon of B6/lpr Mice
Kieran Adam and Adam Mor
Jul 20, 2022 3606 Views

Monitoring Group 2 Innate Lymphoid Cell Biology in Models of Lung Inflammation
Jana H. Badrani [...] Taylor A. Doherty
Jul 20, 2023 2681 Views
Abstract
The ability to assess the function of a range of cytokine, antigen receptor, and Toll-like receptor (TLR) signaling pathways in a range of immune cells could provide a kind of fingerprint of the state of the human immune system. The mass cytometry or CyTOF, platform allows for the parallel application of about 40 labeled antibodies to a single sample, creating the possibility to read out many cell types and signaling pathways in a single small blood sample. We developed such a mass cytometry panel, consisting of 22 antibodies to cell surface lineage markers and 8 antibodies to phospho-specific epitopes of signaling proteins. These antibodies were chosen to discriminate all major white blood cell lineages, to a level of detail that includes subsets such as naïve, central memory, effector memory, and late effector CD4+ and CD8+T cells, naïve, transitional, and switched memory B cells, plasmablasts, myeloid and plasmacytoid dendritic cells, CD16+ and CD16+CD56+ NK cells, CD16+ and classical monocytes etc. 32 such cell subsets are defined in our standard gating scheme. The eight phospho-specific antibodies were chosen to represent major signaling nodes responsive to cytokine, TLR, and antigen receptor signaling. This antibody panel is used with 8 standard stimulation conditions (unstimulated, IFNa, IL-6, IL-7, IL-10, IL-21, LPS, PMA+ ionomycin), although other stimuli can be added. Comparison of healthy controls to subjects with immune deficiencies of unknown etiology may help elucidate the mechanisms of such deficiencies.
Phosphorylation of tyrosine, serine, and threonine residues is critical for the control of protein activity involved in various cellular events. An assortment of kinases and phosphatases regulate intracellular protein phosphorylation in many different cell signaling pathways, such as T and B cell signaling, those regulating apoptosis, growth and cell cycle control, plus those involved with cytokine, chemokine, and stress responses. Phosphoflow assays combine phospho-specific antibodies with the power of flow cytometry to enhance phospho protein study. In our assay, peripheral blood mononuclear cells are stimulated by cytokines, fixed, surface-stained with a cocktail of antibodies labeled with MAXPAR (Brand Name) metal-chelating polymers and permeabilized with methanol. They are then stained with intracellular phospho-specific antibodies.
We use a CyTOFTM mass cytometer to acquire the ICP-MS data. The current mass window selected is approximately AW 103-203, which includes the lanthanides used for most antibody labeling, as well as iridium and rhodium for DNA intercalators. Subsequent analysis of the dual count signal data using FlowJo software allows for cell types to be analyzed based on the dual count signal in each mass channel. The percentage of each cell type is determined and reported as a percent of the parent cell type. Median values are reported to quantitate the level of phosphorylation of each protein in response to stimulation. Comparing the level of phosphorylation between samples can offer insight to the status of the immune system. Whole blood stimulation is the closest to the in vivo condition and it allows for assessment of granulocyte population as well as lymphocytes and monocytes.
Materials and Reagents
- Whole blood from patient or donor
- Cytokine aliquots (IFNα, IL-6, IL-7, IL-10, IL-21, LPS, PMA/Ionomycin etc.)
- IFNa (PBL Interferon source, catalog number: 11105-1 )
- IFNg2 (BD Biosciences, catalog number: 554617 )
- IL6 (BD Biosciences, catalog number: 550071 )
- IL7 (BD Biosciences, catalog number: 554608 )
- IL10 (BD Biosciences, catalog number: 554611 )
- IL21 (Life Technologies, Gibco®, catalog number: PHC0214 )
- IL2 (BD Biosciences, catalog number: 554603 )
- CD3 (BD Biosciences, catalog number: 555329 )
- CD28 (BD Biosciences, catalog number: 555725 )
- LPS (Sigma-Aldrich, catalog number: L7770 )
- IL5 (Pepro Tech, catalog number: 200-05 )
- IL17A (Pepro Tech, catalog number: 200-17 )
- IL17E (Pepro Tech, catalog number: 200-24 )
- IFNa (PBL Interferon source, catalog number: 11105-1 )
- Methanol (Thermo Fisher Scientific, catalog number: A452SK-1 )
- Dulbecco’s Phosphate-buffered saline (Ca2+, Mg2+)
- Phenotyping and phosphoprotein antibodies filtered with 0.1 um spin filters to get even staining of markers
- Ir-intercalator stock solution from Fluidigm Sciences (Rh103-intercalator can be used)
- 10x phosphate-buffered saline (Rockland, catalog number: MB-008 )
- Smart tube 1x thaw-lyse buffer (Smart Tube Inc.)
- Complete RPMI (see Recipes)
- CyFACS buffer (see Recipes)
Equipment
- Nunc Coded Cryobank Vials (Cluster tubes, catalog number: 374078 )
- 37 °C water bath
- Biosafety cabinet
- Centrifuge
- CO2 incubator at 37 °C
- Calibrated pipettes
- 8 or 12 pin aspirator (V&P Scientific, model: Inc VP187A )
- Smart tube proteomic stabilizer (Smart Tube Inc.)
Procedure
- Prepare stimulations in cluster tubes
- Prepare cytokines at 5x concentrations in Complete RPMI, with enough volume to pipette 50 µl into a well for each sample and control. See chart below for dilution for a full plate.
- The Cluster tubes with the aliquoted stimulants can be frozen away at -80 °C until further use.
- Prepare cytokines at 5x concentrations in Complete RPMI, with enough volume to pipette 50 µl into a well for each sample and control. See chart below for dilution for a full plate.
- Example of a full plate
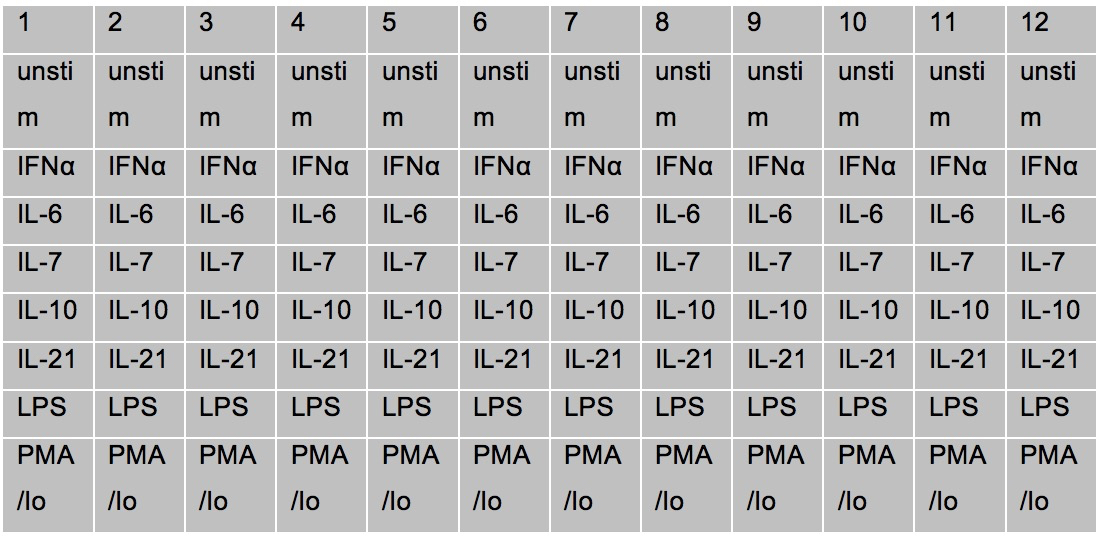
- Example of cytokine stimulations
IFNα: Final concentration of stimulation used=10,000 units/ ml
IFNγ2: Final concentration of stimulation used= 50 ng/ ml
IL6: Final concentration of stimulation used= 50 ng/ ml
IL7: Final concentration of stimulation used= 50 ng/ ml
IL10: Final concentration of stimulation used= 50 ng/ ml
IL21: Final concentration of stimulation used= 50 ng/ ml
IL2: Final concentration of stimulation used= 50 ng/ ml
CD3 = 2.5 μl in 990 ul (Final concentration 500 ng/ml)
CD28 = 10 μl in above media (Final concentration 2,000 ng/ml)
LPS: Final concentration of stimulation used= 1 μg/ ml
PMA: 10 ng/ml final concentration /ml
Ionomycin: 1,000 ng/ml final concentration /ml
IL5: Final concentration of stimulation used= 10 ng/ ml
IL17A: Final concentration of stimulation used= 50 ng/ ml
IL17E: Final concentration of stimulation used= 50 ng/ ml - Stimulation
- Rest the blood collected from donors in incubator at 37 °C in CO2 incubator for 1 h. Just before use, take out the required number of cluster tubes with the stimulant from the -80 °C freezer and let warm at 37 °C water bath for 5-10 min.
- Aliquot 200 μl of whole blood into column 1 of the cluster tube plate using a multichannel pipette. Change tips between each patient.
- Repeat with all the columns of tubes depending on the number of donors.
- Work as rapidly as possible.
- Tap plate to mix, and incubate 15 min at 37 °C in CO2 incubator.
- Remove stimulated whole blood from incubator at 15 min and using a multichannel pipette, add 250 µl of 1x Proteomic Stabilizer to each column of patient samples in the Cluster tube block. Pipette up and down to mix for each patient. Change tips between patients. Add the stabilizer in the same order that you added the whole blood for stimulation.
- Incubate for 10 min at room temperature. At this point it can be frozen away at -80 °C until it is ready to be Thaw-lysed and surface stained.

Figure 1. A flow chart of the steps described in our procedure
- Rest the blood collected from donors in incubator at 37 °C in CO2 incubator for 1 h. Just before use, take out the required number of cluster tubes with the stimulant from the -80 °C freezer and let warm at 37 °C water bath for 5-10 min.
- Surface staining
- Take out the required number of frozen stimulated cluster tubes with whole blood as can be comfortably run on the CyTOF in 1 day. (You can run 3-4 cluster or strip of samples in one day by CyTOF. One cluster or strip has 8 wells). Thaw in cold water for about 15 min.
- Using a pipette, transfer the fixed whole blood sample (450 μl) into a labeled deep well plate with 1.2 ml of 1x Thaw-Lyse and let it sit for 10 min at room temperature.
- Centrifuge cells at 548 rcf (x g) for 10 min at room temperature.
- Aspirate supernatant from the cells.
- Vortex the pellet and add 1.6 ml, 1x Thaw-Lyse, and let it sit for 5-10 min at room temperature. Centrifuge cells at 548 rcf (x g) for 10 min at room temperature.
- Aspirate supernatant from the cells.
- Vortex and wash the pellet with ~1.8 ml CyFACS.
- Centrifuge cells at 974 rcf (x g) for 10 min at 4 °C.
- Aspirate supernatant from the cells so that about 50 ul remains at the bottom of each well.
- Make cocktail in phosphate buffered saline of metal-chelating polymer-labeled surface antibodies according to previously determined titration. Make sufficient volume for each well to have 20 μl of cocktail. Pipet into 0.1 μm spin filter and centrifuge in a tabletop microcentrifuge (RCF=14,000) for 2 min at room temperature. This ensures even staining.
- Add 20 μl of antibody cocktail to the cells in the deep well plate, vortex to mix and let it incubate at room temperature for 30 min.
- Wash cells with 1.8 ml phosphate buffered saline and centrifuge cells at 974 rcf (x g) for 10 min at 4 °C. Aspirate.
- Permeabilize the cells by adding 600 µl cold methanol to each well of the deep well block using a multichannel pipette. Pipette up and down to mix for each patient. Change tips between patients. Cells are stored overnight at this point at -80 °C. This is done for convenience of work flow.
- Remove samples from freezer. Add 1.0 ml of CyFACS. Centrifuge cells at 974 rcf (x g) for 10 min at 4 °C. Aspirate so that about 100 μl remains in the wells.
- Wash in 1.8 ml/ well CyFACS buffer.
- Centrifuge cells at 974 rcf (x g) for 10 min at 4 °C. Discard supernatant by aspiration.
- Take out the required number of frozen stimulated cluster tubes with whole blood as can be comfortably run on the CyTOF in 1 day. (You can run 3-4 cluster or strip of samples in one day by CyTOF. One cluster or strip has 8 wells). Thaw in cold water for about 15 min.
- Intracellular staining
- Make cocktail in PBS of metal-chelating polymer-labeled intracellular antibodies according to previously determined titration. Make sufficient volume for each sample to have 20 μl of cocktail. Pipet into 0.1 μm spin filter and centrifuge in tabletop microcentrifuge (RCF=14,000) for 2 min at room temperature.
- Add 20 μl of antibody cocktail to the cells in the deep well plate, vortex to mix and let it incubate at room temperature for 30 min.
- Add 1.6 ml of PBS. Centrifuge cells at 974 rcf (x g) for 10 min at 4 °C. Discard supernatant by aspiration.
- Make 1:200 dilution in PBS of Ir-intercalator. Add 20 μl of diluted Ir-intercalator solution to each sample, pipet to mix. Incubate on ice for 20 min.
- Wash 3 times in MilliQ water. Centrifuge cells at 974 rcf (x g) for 10 min at 4 °C. Discard supernatant by aspiration.
- Bring up the samples in 1 ml of MilliQ water and filter samples through a cell strainer. Acquire samples on the Cytof, after standard instrument setup procedures.
- Make cocktail in PBS of metal-chelating polymer-labeled intracellular antibodies according to previously determined titration. Make sufficient volume for each sample to have 20 μl of cocktail. Pipet into 0.1 μm spin filter and centrifuge in tabletop microcentrifuge (RCF=14,000) for 2 min at room temperature.
Representative data
Table 1. Antibodies in the phospho-CyTOF panel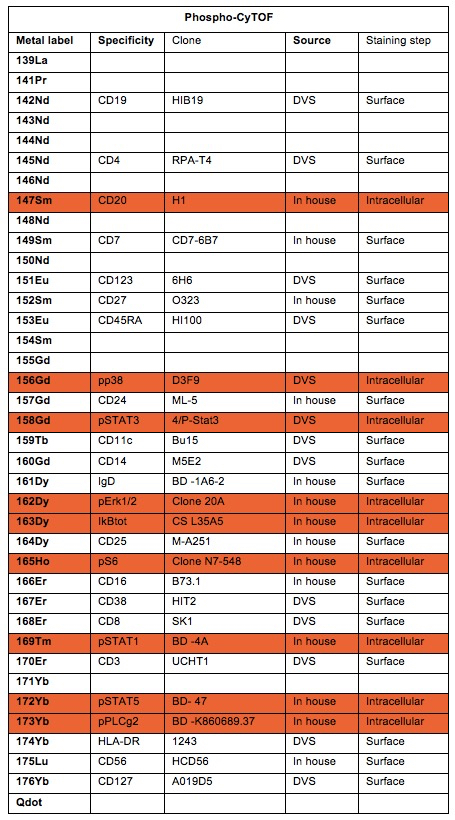
Whole Blood Gating Scheme
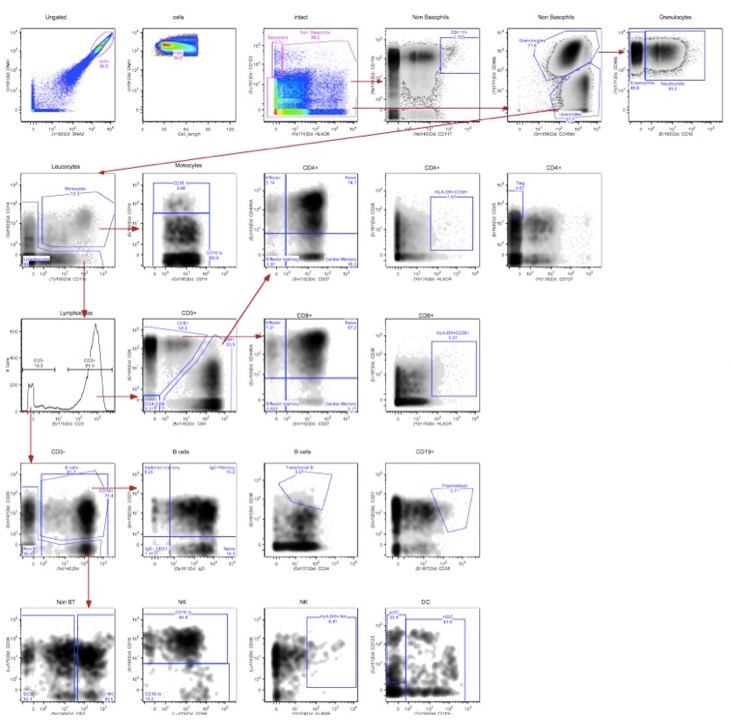
Figure 2. Whole blood gating scheme
Notes
- Staining volumes are kept at a total of 50 to 60 μl.
- Washing steps are critical to minimize background. Aspirate such that only ~ 100 μl volume is left after each wash. Vortex before the next wash.
- Homogeneous staining is aided by thorough resuspension via pipetting up and down.
- Sample dilution is critical to collecting an optimal number of single cell events; aim for about 300 total events/sec.
- Do not leave sample buffer or wash buffers exposed to air, as dust accumulation can cause clogs.
- Each sample should be resuspended in water and filtered through nylon mesh just prior to running; do not let samples sit on water, as cells will lyse and clump, causing clogs and loss of events.
Recipes
- Complete RPMI (Hyclone RPMI-1640 Medium)
RPMI with 10% FBS
Penicillin/streptomycin
Glutamine - CyFACS buffer
1x CyPBS PBS with 0.1% BSA, and 0.05% Na azide
Made in MilliQ water
Note: Do not use FBS!
Acknowledgments
This work was supported by grants S10RR027582, 5U19AI057229, and 5U19AI090019 from the U.S. National Institutes of Health.
References
- Bendall, S. C., Simonds, E. F., Qiu, P., Amir el, A. D., Krutzik, P. O., Finck, R., Bruggner, R. V., Melamed, R., Trejo, A., Ornatsky, O. I., Balderas, R. S., Plevritis, S. K., Sachs, K., Pe'er, D., Tanner, S. D. and Nolan, G. P. (2011). Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332(6030): 687-696.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Fernandez, R. and Maecker, H. T. (2015). Cytokine-Stimulated Phosphoflow of Whole Blood Using CyTOF Mass Cytometry. Bio-protocol 5(11): e1495. DOI: 10.21769/BioProtoc.1495.
Category
Immunology > Immune cell staining > Immunodetection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










