- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and 3-dimensional Culture of Primary Murine Intestinal Epithelial Cells
Published: Vol 4, Iss 10, May 20, 2014 DOI: 10.21769/BioProtoc.1125 Views: 26670
Reviewed by: Lin FangFanglian He

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1251 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1460 Views

Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
Adriana Bajetto [...] Tullio Florio
Feb 5, 2026 92 Views
Abstract
The intestine, together with skin and blood, belongs to the organs with the highest cell turnover, which makes it a perfect model to study cellular processes, such as proliferation and differentiation. Epithelial cell turnover in intestine is possible due to the presence of intestinal stem cells, which are located at the bottom of the crypt. Here, we recapitulate a detailed protocol for the isolation and culture procedures of primary epithelial intestinal cells in a three - dimensional (3D) in vitro system, described for the first time by Hans Clevers group (Sato et al., 2009). This specific 3D culture preserves intestinal stem cells, which give rise to differentiated progeny for example goblet cells. The culture has many applications and represents a useful model to study stem cell biology, epithelial cell regeneration, and transplantation studies. Moreover, the presented 3D culture can be used to investigate the barrier function of intestinal epithelial cells, as well as heterotypic cell interactions between epithelial cells and stromal cells.
Keywords: Intestinal epitheliumMaterials and Reagents
- Mouse (4 weeks-16 weeks old)
- Advanced DMEM/F12 (Life Technologies, Gibco®, catalog number: 12634-010 )
- Heat Inactivated Fetal Bovine Serum (FBS) (Life Technologies, Gibco®, catalog number: 10500-064 )
- PBS without Ca2+ and Mg2+ (Life Technologies, Gibco®, catalog number: 14190-094 )
- Penicillin/Streptomycin (10,000 Units/ml Penicillin; 10,000 µg/ml Streptomycin) (Life Technologies, Gibco®, catalog number: 15140-22 )
- 70% ethanol
- 100x GlutaMAXTM-I (Life Technologies, Gibco®, catalog number: 35050-038 )
- 1 M HEPES (Life Technologies, Gibco®, catalog number: 15630-080 )
- 50x B-27 Supplement (Life Technologies, Gibco®, catalog number: 17504-044 )
- 100x N-2 Supplement (Life Technologies, Gibco®, catalog number: 17502-048 )
- BD Matrigel Basement Membrane Matrix (10 ml) (BD Biosciences, catalog number: 354234 )
- Gentamicin Reagent Solution (50 mg/ml) (Life Technologies, catalog number: 15750-060 )
- Sterile 0.1% BSA (in PBS)
- Sterile 2 mM EDTA (see Recipes)
- Washing solution (see Recipes)
- N-Acetyl-L-cysteine (Sigma-Aldrich, catalog number: A9165-5G ) (see Recipes)
- Recombinant Murine EGF (Pepro Tech, catalog number: AF-315-09 ) (see Recipes)
- Recombinant Murine Noggin (Pepro Tech, catalog number: 250-38 ) (see Recipes)
- Recombinant Human R-Spondin-1 (Pepro Tech, catalog number: 120-38 ) (see Recipes)
Equipment
- Forceps and short, sharp-point scissors (e.g. Hardened Fine Iris Scissors, Fine Science Tools, catalog number: 14090-09 )
- 70 µm cell strainer (BD Biosciences, Falcon®, catalog number: 352350 )
- Centrifuge 5702R (Eppendorf)
- Cover glass
- Microscope
- 24-well tissue culture plate (Sarstedt AG, catalog number: 83.1836 )
- Petri dishes
- Falcon tubes (50 ml, 15 ml)
- Pipettes (20 ml, 10 ml, 1 ml, 100 µl)
- Pipetboy
- 37 °C, 5% CO2 cell culture incubator
Procedure
Part I. Isolation
Before starting: put matrigel on ice in order to thaw it.
Note: For the matrigel handling, please follow the manufacturer’s instructions.
- Harvesting
- The small intestine is harvested from a mouse.
- The intestine is placed in a Petri dish containing ice-cold washing solution and then fat and the adjacent tissue are removed.
Note: Both tissue and Washing solution should be stored on ice during the whole procedure.
- The intestine is opened lengthwise and washed out from the luminal contents using washing solution.
- The villi of an intestine are scraped off using a cover glass.
Note: From this step the procedure should be continued under sterile conditions. To be on a safe side it is recommended to perform scraping on both sides of an intestine. Detached villi are seen as cloudy solution.
- The intestine is washed with washing solution and then cut with sharp-point scissors into 2-4 mm pieces.
- The material is transferred into a 50 ml falcon tube containing 10-20 ml of washing solution.
Note: Tissue fragments might stick to the pipette. To avoid this, pre-wetting of the pipette is recommended.
- The material is pipetted up and down a few times with a 10 ml pipette.
- After sedimentation of tissue fragments, the supernatant is removed.
- The small intestine is harvested from a mouse.
- Washing
- For the washing, 10-20 ml of washing solution is added. Then, tissue fragments are sedimented. After that, the supernatant is removed.
- Step B1 is being repeated until the supernatant is clear (approximately 10 times).
- 25 ml of 2 mM EDTA is added to the tissue fragments.
- The falcon tube that contains tissue fragments and EDTA is placed on a shaker and incubated for 15 min at 4 °C.
- Tissue fragments are sedimented followed by the removal of the supernatant.
- 10-20 ml of washing solution is added. Then, tissue fragments are sedimented. After that, the supernatant is removed.
- The Washing solution is added to the tissue fragments, pipetted up and down 3-5 times and passed through a 70 µm cell strainer.
- The flow-through (I fraction) is stored on ice and will be further proceeded in step C3 of Elution.
Note: Crypts are present in the supernatant and go through the pores of the cell strainer, whereas the tissue fragments stay on the top of the cell strainer.
- Tissue fragments that remained on the cell strainer are proceeded further.
- On a pipette tip the tissue fragments are transferred into a 50 ml falcon tube containing 25 ml of 2 mM EDTA.
- The falcon tube is placed on a shaker and incubated for 30 min at 4 °C.
- For the washing, 10-20 ml of washing solution is added. Then, tissue fragments are sedimented. After that, the supernatant is removed.
- Elution
- After tissue sedimentation, the supernatant is discarded.
- Washing solution is added to the tissue fragments, gently pipetted up and down 3-5 times and passed through a 70 µm cell strainer (II fraction).
Note: This step is repeated; as a result fraction III will be collected (optional). Tissue fragments might stick to the pipette. To avoid that, pre-wetting of the pipette is recommended.
- Crypts from certain fractions are observed under the microscope. Presence of finger-like structures (= crypts, see Figure 1) indicates a successful isolation.
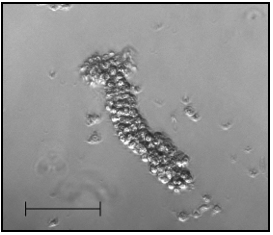
Figure 1. A representative of a freshly isolated crypt. Objective 10x, scale bar 100 µm
- Crypts are spin down at 600 rpm for 5 min at 4 °C to remove single cells. A 24-well plate is pre-warmed by placing it into the cell culture incubator.
- After centrifugation the supernatant is discarded.
Note: If the pellet is not visible, the centrifugation should be repeated.
- The pellet is resuspended in 10 ml of ice-cold Basal Medium 2 (BM2; see Table 1). Crypts are counted under the microscope.
Table 1. Composition of BM2 (50 ml)Stored at 4 °C or -20 °C (avoid repeated freeze thaw cycles)Component
Volume
50x B27100x N2N-Acetyl-L-cysteine (500 mM)1 ml0.5 ml125 µlBM1 (see Table 2)
To final volume of 50 ml
- The crypts are centrifuged at 800 rpm for 5 min at 4 °C.
- Crypt pellet is resuspended in matrigel:
- Use 100-500 crypts per 50 µl of matrigel
- Pipette gently, avoid bubbles
- Use 100-500 crypts per 50 µl of matrigel
- 50 µl of matrigel is pipetted per well into the pre-warmed 24-well plate and then the plate is left for 2-3 min at room temperature.
Note: Pipette matrigel exactly in the middle of the well, so that as a result matrigel drop is formed (see Figure 2).
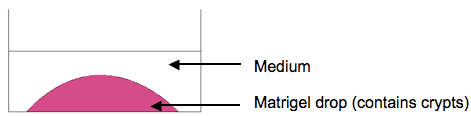
Figure 2. Scheme of the organoid culture
- The plate is transferred into the cell culture incubator and incubated at 37 °C for 5-10 min (until the matrigel solidifies completely).
- 0.5 ml of crypt culture medium (CCM; see Table 3) is added per well.
- The plate is transferred back to the cell culture incubator.
- After tissue sedimentation, the supernatant is discarded.
Part II. Culture
2-3 times per week the medium is removed and 0,5 ml/well of fresh medium (CCM, see Table 3) is added. When organoids are big (see Figure 3C), they should be passed. Usually the passage is performed once a week. Organoids should be splitted in 1:4 ratio.
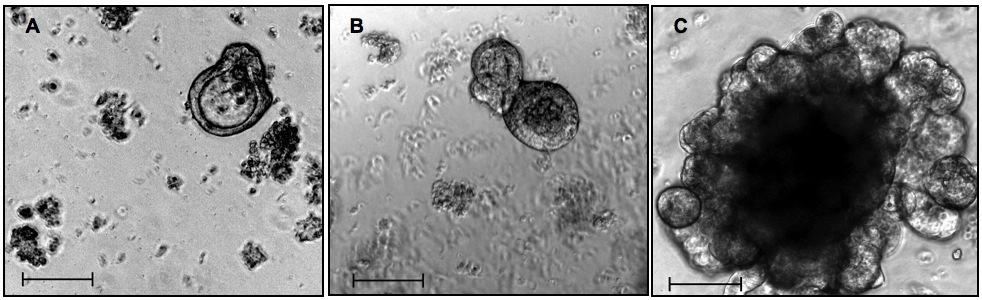
Figure 3. Isolated small intestinal crypts in 3D culture. A, B, C - 48 h/5 days/12 days in culture, respectively. A and B, passage 0. C, passage 1. Phase contrast, objective 10x, scale bars 100 µm
Passage procedure:
- Medium is removed.
- 0.5 ml of ice-cold Basal medium 1 (see Table 1) well is added.
- Plate is incubated on ice for at least 5 min.
Note: Starting from this step organoids will be kept on ice for the whole time.
- Organoids are pipetted up and down several times (1 ml pipette is used.).
- All organoids are transferred into one ice-cold 15 ml falcon tube and fill up with Basal Medium 1 (BM1; see Table 2) to final volume of 15 ml.
Table 2. Composition of BM1 (500 ml)Stored at 4 °CComponent
Volume
Advanced DMEM/F12100x GlutaMax100x Pen/StrepHepes (1 M)485 ml5 ml5 ml5 ml
- Organoids are centrifuged for 600 rpm for 5 min at 4 °C. During centrifugation a 24-well plate is pre-warmed at 37 °C.
- The supernatant is carefully removed.
Note: Remove as much supernatant as possible. Be careful not to remove organoids.
- 10 ml of ice-cold BM1 is added to the organoid pellet.
- The organoids are centrifuged for 800 rpm for 5 min at 4 °C.
- The supernatant is carefully removed.
Note: During this step nice pellet should be visible and the supernatant should be removed completely.
- The organoids are mixed with matrigel and pipetted up and down.
Note: Avoid bubbles during pipetting. Use 4x volume of matrigel used for the first time.
- 50 µl matrigel/well is pipetted into the pre-warmed 24-well plate.
Note: Pipette matrigel exactly in the middle of the well, so that as a result matrigel drop is formed.
- The plate is left for 2-3 min at room temperature.
- The plate is transferred into the cell culture incubator and incubated at 37 °C for 5-10 min (until the matrigel solidifies completely).
- 0.5 ml of CCM (see Table 3) is added per well.
- The plate is transferred back to the cell culture incubator.
Table 3. Composition of CCM (20 ml)Stored short-term at 4 °C or -20 °C (avoid repeated freeze thaw cycles)Component
Volume
BM2EGF (50 ng/µl)Noggin (100 ng/µl)R-Spondin-1 (1 µg/µl)20 ml10 µl20 µl10 µl
Notes
- The percentage of viable crypts in the culture is approximately 90-95%. Crypt viability in vitro strictly depends on the quality of the growth factors used for preparation of the culture medium. Moreover, medium should be either freshly prepared (and stored at 4 °C up to 5 days) or single-use aliquots should be made and stored at -20 °C for one month.
- Viable and healthy organoid culture is characterized by the presence of buds and increase in organoid size over the time. Just after the passage, organoids are small, but then they grow again into structures as seen in the Figure 3C. Crypt culture contains intestinal stem cells, which represent a multipotent stem cell population, as they have potential to differentatiate only into intestinal epithelial cells. There are certain types of intestinal epithelial cells and they can be identified by stainings: stem cells (e.g. Lgr5 staining), Goblet cells (e.g. periodic acid-Schiff staining), Paneth cells (e.g. lyzozyme staining), enterocytes (e.g. intestinal alkaline phosphatase staining), enteroendocrine cells (e.g. chromogranin A staining) (Sato et al., 2009). Some other possible stainings are: b-catenin (active Wnt signaling) and Ki-67 (proliferation marker).
Recipes
- 2 mM EDTA
500 ml PBS
2.5 ml 0.5 M EDTA (pH 8) in distilled water (sterile filtered)
Stored at 4 °C
- Washing solution
50 ml FBS
450 ml PBS
0.5 ml gentamicin, stock 50 mg/ml (optional)
Stored at 4 °C
- 500 mM N-Acetyl-L-cysteine
Dissolve 407.5 mg of N-Acetyl-L-cysteine in 5 ml of distilled water
Sterile filter
Make qliquots
Stored at 4 °C
- EGF, Noggin, R-Spondin-1
Prepare stock solutions (EGF 50 ng/µl; Noggin 100 ng/µl; R-Spondin 1 µg/µl) according to the manufacter´s instructions
Example:
Noggin 100 ng/µl
Add 1 ml of 0.1% BSA (in PBS) into a vial with 100 µg Noggin
Mix by pipetting
Make aliquots
Stored at -20 °C
Acknowledgments
This protocol was adapted from Sato et al. (2009).
References
- Sato, T., Vries, R. G., Snippert, H. J., van de Wetering, M., Barker, N., Stange, D. E., van Es, J. H., Abo, A., Kujala, P., Peters, P. J. and Clevers, H. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244): 262-265.
Article Information
Copyright
© 2014 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Pastuła, A. and Quante, M. (2014). Isolation and 3-dimensional Culture of Primary Murine Intestinal Epithelial Cells. Bio-protocol 4(10): e1125. DOI: 10.21769/BioProtoc.1125.
Category
Cell Biology > Cell isolation and culture > 3D cell culture
Stem Cell > Adult stem cell > Epithelial stem cell
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










