- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Acquisition of Leftward Flow in Xenopus laevis
Published: Vol 3, Iss 23, Dec 5, 2013 DOI: 10.21769/BioProtoc.996 Views: 9649
Reviewed by: Ru Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Immunolocalization of Proteins in Corals: the V-type H+-ATPase Proton Pump
Katie L. Barott and Martin Tresguerres
Sep 5, 2015 11066 Views

Rapid and solvent-free, 2-hydroxyethyl methacrylate (HEMA)-acrylamide (AAm) copolymer-based optical clearing of tissue for fluorescent imaging
Yanran Wang [...] Kefeng Wu
Nov 20, 2025 1509 Views

Characterizing Tissue Oxygen Tension During Neurogenesis in Human Cerebral Organoids
Yuan-Hsuan Liu and Hsiao-Mei Wu
Nov 20, 2025 1704 Views
Abstract
In Xenopus, the left-right axis is established following an extracellular vectorial leftward flow driven by monocilia at the gastrocoel roof plate (GRP) during late gastrulation / neurulation (Schweickert et al., 2007). As the GRP lies inside the developing archenteron, imaging of flow is challenging. Here we present the detailed procedure to visualize leftward flow in Xenopus laevis embryos.
Keywords: CiliaMaterials and Reagents
- Fluorescent micro beads (e.g. FluoSpheres 0.5 μm) (Life Technologies, catalog number: F-8813 )
- Agarose
- Penicillin/Streptomycin (10.000 Units/ml; Gibco®, catalog number: 15140 )
- HEPES Pufferan (Carl Roth, catalog number: HN78.3 )
- Bead solution (see Recipes)
- 5x MBSH (Modified Barth‘s Saline) (see Recipes)
Equipment
- Sharp forceps (Fine Science Tools, catalog number: Dumont #5 )
- Micro blade (Fine Science Tools, Dissecting Knife - Fine Tip)
- Sectioning dish (Petri Dish with bottom covered in 1% Agarose in 1x MBSH)
- Transfer pipet (Carl Roth, model: EA65.1 )
- Glass staining block (Karl-Hecht Assistent (Lymphbecken) 2020)
- Microscope slides
- Coverslips
- 5 ml syringe
- Vaseline
- Fluorescence microscope equipped with a digital camera (wide field)
- Large Petri dish filled with PBS or MBSH for explant retrieval
Procedure
- Raise Xenopus embryos to stage 17 (Nieuwkoop et al., 1994).
- Build a flexible imaging chamber (Figure 1) by putting the opening of a vaseline filled syringe directly onto a microscope slide. Gently press the piston to release vaseline as you draw a rectangle (approximately 1.5 x 1 cm) onto the slide. Make sure that the rectangle is closed. Fill the rectangle with the bead solution until it has a convex meniscus.
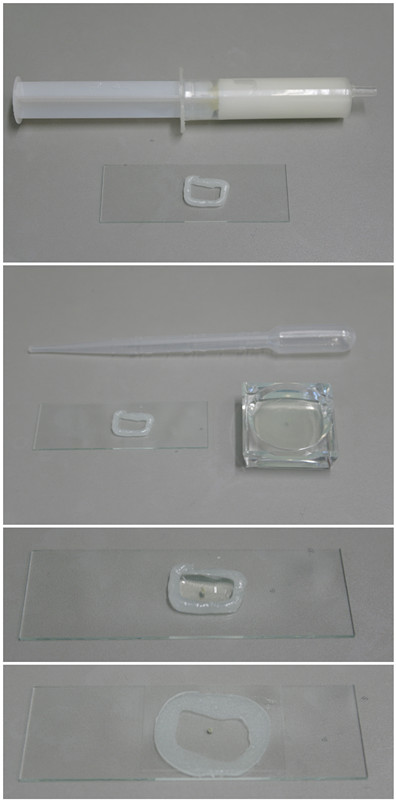
Figure 1. A flexible imaging chamber
- Cut dorsal explant (cf. Figure 2 in Blum et al., 2009):
- Transfer a stage 17 embryo into a sectioning Petri dish filled with 1x MBSH.
- With a micro blade, remove the head by transversally cutting the anterior part of the embryo.
- Erect the cup-shaped embryo such that you can see inside. Then place small alternating cuts at the left and right ‘lateral lines’ until you reach the area of the circumblastoporal collar.
- With forceps and the micro blade, pull apart the dorsal and ventral halves of the embryo until you see the opening of the blastopore from the inside.
- Place a final cut ventral to the blastopore to separate the dorsal explant from the rest of the embryo. Make sure not to touch the dorsal explant with the forceps or blade from the inside.
- You should now be left with a bathtub shaped dorsal explant with the GRP placed at its deepest point.
- Transfer a stage 17 embryo into a sectioning Petri dish filled with 1x MBSH.
- With a transfer pipet, carefully bring the dorsal explant into the glass staining block that contains the FluoSphere bead solution and gently pipet the explant up and down in this medium to make sure that FluoSpheres reach the GRP.
- Transfer the dorsal explant into the FluoSphere solution filled chamber by carefully pipetting. Make sure that the explant never touches the surface of any liquid as surface tension disintegrates the tissue. Orient the explant with the inside facing up, i.e. towards you.
- Seal the flexible chamber with a coverslip by gently pushing it down with opened forceps. Carefully push until the coverslip touches the rim of the dorsal explant, i.e. the left and right ‘lateral line’ and the ventral side of the circumblastoporal collar.
- While pushing down, sometimes the ventral circumblastoporal collar folds over and blocks the GRP from view. If that happens, a slight drag of the coverslip towards the posterior pole can shear the explant to allow imaging of the GRP.
- Let the setup rest for 5-10 min to allow the explant to deform, which otherwise would suggest false particle movements during early acquisition.
- Put the slide in your (wide-field) fluorescence microscope and focus on the GRP.
- Excite with the required wavelength of the FluoSpheres - you should see a ‘starry sky’. Now adjust focus to the focal plane slightly above the GRP.
Note: as the GRP cilia are just 5 μm in length, leftward flow occurs only in the plane right above the epithelium (Schweickert et al., 2007). To facilitate finding the right plane, focus into the tissue until you do not see fluorescence of any bead. Now carefully change the focal plane until the first beads appear in focus.
- In wild type embryos, you should see particles moving to the left of the GRP. In Xenopus laevis, leftward flow is relatively slow (~2.5 μm/s) and therefore best visualized using time-lapse videography (Schweickert et al., 2007). Depending on further analysis and hence temporal resolution, acquire a movie with at least 2 fps.
- For retrieving the dorsal explant after investigation, submerge the complete slide in a large Petri dish filled with 1x MBSH or 1x PBS and carefully remove the coverslip with forceps. The explant should float into the buffer from which it can be pipetted into fixative for further analysis (in situ hybridization, immunohistochemical staining, etc.).
Recipes
- Bead solution
Dilute FluoSpheres 1:2,500 in 1x MBSH (prepare ~5 ml)
- 5 x MBSH (1 L)
Note: Before usage, dilute to 1x with H2O
25.7 g NaCl
0.375 g KCl
1 g NaHCO3
1 g MgSO4.7H2O
0.39 g (CaNO3)2.4H2O
0.3 g CaCl2.2H2O
11.9 g Hepes
5 ml penicillin/streptomycin
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft grants to M.B.
References
- Blum, M., Beyer, T., Weber, T., Vick, P., Andre, P., Bitzer, E. and Schweickert, A. (2009). Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev Dyn 238(6): 1215-1225.
- Nieuwkoop, P. and Faber, J. Normal Table of Xenopus laevis (Daudin), 1994, GARLAND PUBLISHING, New York&London, USA.
- Schweickert, A., Weber, T., Beyer, T., Vick, P., Bogusch, S., Feistel, K. and Blum, M. (2007). Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol 17(1): 60-66.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Thumberger, T. and Blum, M. (2013). Acquisition of Leftward Flow in Xenopus laevis. Bio-protocol 3(23): e996. DOI: 10.21769/BioProtoc.996.
Category
Developmental Biology > Morphogenesis > Motility
Cell Biology > Cell imaging > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










