- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells
Published: Vol 3, Iss 21, Nov 5, 2013 DOI: 10.21769/BioProtoc.960 Views: 17538
Reviewed by: Lin FangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Human Bone Marrow Non-hematopoietic Cells for Single-cell RNA Sequencing
Hongzhe Li [...] Stefan Scheding
Jun 20, 2024 2556 Views

Versatile Click Chemistry-based Approaches to Illuminate DNA and RNA G-Quadruplexes in Human Cells
Angélique Pipier and David Monchaud
Feb 5, 2025 2532 Views

Accurate Identification of Cell Cycle Stages in RPE1 Cells Using the ImmunoCellCycle-ID Method
Syon Reddy [...] Aussie Suzuki
Aug 5, 2025 1870 Views
Abstract
Bromodeoxyuridine (BrdU) is a thymidine analog that is incorporated into DNA during the S-phase of the cell cycle. As such, BrdU incorporation can be used to quantify the number of cells that are in S-phase in the time period during which BrdU is available. The following protocol describes an in vivo BrdU incorporation assay as a measure of cell proliferation in adult murine hematopioetic stem cells (HSCs). Specifically, BrdU incorporation was analyzed for long-term HSCs (LT-HSCs, Lin-Sca-1+c-Kit+CD34-CD135-), Short-term HSCs (ST-HSCs, Lin-Sca-1+c-Kit+CD34+CD135-) and multipotent progenitors (MPPs, Lin-Sca-1+c-Kit+CD34+CD135+) population.
Materials and Reagents
- Mouse
- BrdU
- RPMI1640
- Fetal Bovine Serum (FBS)
- Potassium bicarbonate
- Ammonium chloride
- EDTA
- BSA
- Lineage Cell Depletion Kit (including Biotin-Antibody Cocktail and Anti-Biotin MicroBeads) (Miltenyi Biotec, catalog number: 130-090-858 )
- BD Cytofix/Cytoperm Buffer (Becton, Dickinson and Company, catalog number: 554714 )
- BD Perm/Wash Buffer
- DNase (included in FITC BrdU Flow Kit) (Becton, Dickinson and Company, catalog number: 559619 )
- DPBS without calcium, magnesium (diluted from 10x DPBS) (Hyclone, catalog number: SH 30258.01 )
- Antibodies:
PE-conjugated-Sca-I (Becton, Dickinson and Company, catalog number: 553336 )
APC-conjugated-c-Kit (Becton, Dickinson and Company, catalog number: 553356 )
PerCP-eFluor 710-conjugated-CD135 (eBioscience, catalog number: 46-1351-80 )
eFluor 450-conjugated CD34 (eBioscience, catalog number: 48-0341-80 )
BrdU-FITC (included in FITC BrdU Flow Kit) (Becton, Dickinson and Company, catalog number: 559619)
- Buffer A (see Recipes)
- Red blood cell lysis buffer (see Recipes)
- Staining buffer (see Recipes)
Equipment
- Small scissors and forceps
- 60 mm tissue culture dish
- 23 G needle
- 3 cc syringe
- 15 ml centrifuge tube
- Cell strainer (Becton, Dickinson and Company, catalog number: 352340 )
- Hemacytometer
- MACS MS column (Miltenyi Biotec, catalog number: 130-042-201 )
- MiniMASC separator (Miltenyi Biotec, catalog number: 130-042-102 )
- Centrifuge
- BD LSRFortessa Analytical Flow Cytometer
Procedure
- Animal injection
i.p. Injection of BrdU (10 mg/ml) 100 μl to 8-12 weeks old mouse (50 μg/g BW, so 5 μl/g of BW), after 6 h, inject the second dose. 2 h post 2nd injection, euthanize the mice using CO2 method followed by cervical dislocation, obtain the bone marrow (BM) cells from 2 tibias and 2 femurs. Two injections ensure that BrdU can incorporate to DNA of both slow and quick turnover cells.
- Obtain total bone marrow (BM) cells
- Use small scissors and forceps, dissect out femurs and tibias from mice and place them in a 60 mm tissue culture dish containing 6 ml ice-cold RPMI1640 with 5% heat inactivated FBS. Use Kimwipe tissue to remove muscle and other tissues. Cut off both ends of each bone shaft in the dish.
- Connect the end of the bone with 23 G needle on 3 cc syringe, flush out bone marrow with RPMI1640 with 5% heat inactivated FBS into the dish. Disaggregate bone marrow tissues by repeated aspirations using the same needle. Transfer the cell suspension to 15 ml centrifuge tube.
- Spin down the cells at 350 x g for 5 min at room temperature, remove the supernatant, resuspend the cells in 1 ml of room temperature red blood cell lysis buffer and incubate at room temperature for 5 min, then add 5-10 ml of RPMI 1640 with 5% heat inactivated FBS.
- Pass the cells through a cell strainer. Collect the flow through to a new tube. Take an aliquot and count the cells in a hemacytometer. Spin down at 350 x g for 5 min at room temperature. Remove the supernatant; the cell pellet should not contain any red color. Disaggregate the cell pellet and wash the cells one time with buffer A, spin down at 350 x g for 5 min at room temperature.
- Use small scissors and forceps, dissect out femurs and tibias from mice and place them in a 60 mm tissue culture dish containing 6 ml ice-cold RPMI1640 with 5% heat inactivated FBS. Use Kimwipe tissue to remove muscle and other tissues. Cut off both ends of each bone shaft in the dish.
- Lineage cell staining (follow the mouse Lineage Cell Depletion Kit)
- Resuspend the total BM cells in buffer A (40 μl/107 cells).
- Add Biotin-Antibody Cocktail (10 μl/107 cells) to stain the lineage differentiated cells. Cocktail of biotin-conjugated monoclonal antibodies contains anti-CD5, anti-CD45R(B220), anti-CD11b, anti-Gr-1(Ly-6G/C), anti-Neutrophil (7/4) and anti-Ter-119.
- Mix well and incubate for 10 min at 4 °C.
- Add additional buffer A in media (30 μl/107 cells) then add Anti-Biotin MicroBeads (20 μl/107 cells, provided in Lineage Cell Depletion Kit).
- Mix well and incubate for 15 min at 4 °C.
- Wash cell by adding 2 ml of buffer A. Centrifuge at 300 x g for 10 min at room temperature.
- Remove the supernatant and resuspend the pellet in 0.5 ml of buffer A.
- Resuspend the total BM cells in buffer A (40 μl/107 cells).
- Lineage depleation
- Place MACS MS column in MiniMASC separator.
- Prepare column by rinsing with 0.5 ml buffer A.
- Apply cell suspension onto the column. Allow the cells to pass through and collect flow through as Lin- fraction.
- Wash column 3 times with buffer A (0.5 ml/each), wash each time once the column reservoir is empty.
- Collect all the elute (Lin-) in one tube.
- Count the Lin- cells, aliquot 1.5 x 106 cells to a new tube.
- Add 2 times more staining buffer to the cell suspension.
- Centrifuge the cells at 350 x g for 5 min.
- Place MACS MS column in MiniMASC separator.
- Stain the Lin- cells with surface antigens:
Test Total (μl) Percp-eFluor 710 eFluor 450 PE APC Staining
bufferCD135 CD34 Sca-I CD117
(c-kit)1 75 1 2 0.5 0.5 71 - Stain each test sample per 1.5 x 106 cells/75 μl buffer, make antibody mix as following, for more samples, increase antibody amount and staining buffer proportionally.
- Add 75 μl of antibody mix to the cell pellet. Incubate cells with antibodies for 15 minutes at room temperature (protected from light).
- Wash one time with staining buffer. Spin down for 5 minutes at 350 x g, and discard the supernatant.
- Stain each test sample per 1.5 x 106 cells/75 μl buffer, make antibody mix as following, for more samples, increase antibody amount and staining buffer proportionally.
- Fix and permeabilize the cells
- Resuspend the cells in 100 μl of BD Cytofix/Cytoperm Buffer per tube.
- Incubate the cells for 15 to 30 minutes at room temperature or on ice.
- Wash the cells with 1 ml of 1x BD Perm/Wash Buffer (dilute the 10x buffer with deionized H2O). Centrifuge at 350 x g for 5 minutes at room temperature, and discard the supernatant.
- Resuspend the cells in 100 μl of BD Cytofix/Cytoperm Buffer per tube.
- Enhance the permeabilization:
- Resuspend the cells in 100 μl of BD Cytoperm Permeabilization Buffer Plus per tube. This reagent is specially formulated for the BrdU Flow kit and is used as a staining enhancer and secondary permeabilization reagent.
- Incubate the cells for 10 minutes on ice.
- Wash the cells in 1 ml of 1x BD Perm/Wash Buffer (as in step 5c).
- Resuspend the cells in 100 μl of BD Cytoperm Permeabilization Buffer Plus per tube. This reagent is specially formulated for the BrdU Flow kit and is used as a staining enhancer and secondary permeabilization reagent.
- Re-fix cells after secondary permeabilization:
- Resuspend the cells in 100 μl of BD Cytofix/Cytoperm Buffer per tube.
- Incubate the cells for 5 minutes at room temperature or on ice.
- Wash the cells in 1 ml of 1x BD Perm/Wash Buffer (as in step 5c).
- Resuspend the cells in 100 μl of BD Cytofix/Cytoperm Buffer per tube.
- Treat with DNase to expose incorporated BrdU
- Resuspend the cells in 100 μl of diluted DNase (diluted to 300 μg/ml in DPBS) per tube, (i.e. 30 μg of DNase/106 cells).
- Incubate cells for 1 hour at 37 °C.
- Wash the cells in 1 ml of 1x BD Perm/Wash Buffer (as in step 5c).
- Resuspend the cells in 100 μl of diluted DNase (diluted to 300 μg/ml in DPBS) per tube, (i.e. 30 μg of DNase/106 cells).
- BrdU intracellular antigens staining
- Make diluted BrdU antibody (1 μl to 50 μl/sample in BD Perm/Wash Buffer).
- Incubate the cells for 20 minutes at room temperature.
- Wash the cells in 1 ml of 1x BD Perm/Wash Buffer (as in step 4c).
- Make diluted BrdU antibody (1 μl to 50 μl/sample in BD Perm/Wash Buffer).
- Resuspend the cells in 0.3 ml of staining buffer and perform flow cytometry analysis. Samples can be stored overnight at 4 °C, protected from light, prior to analysis by flow cytometry.
- Flow cytometry was performed on BD LSRFortessa Analytical Flow Cytometer with the following gating strategy (Figure 1).
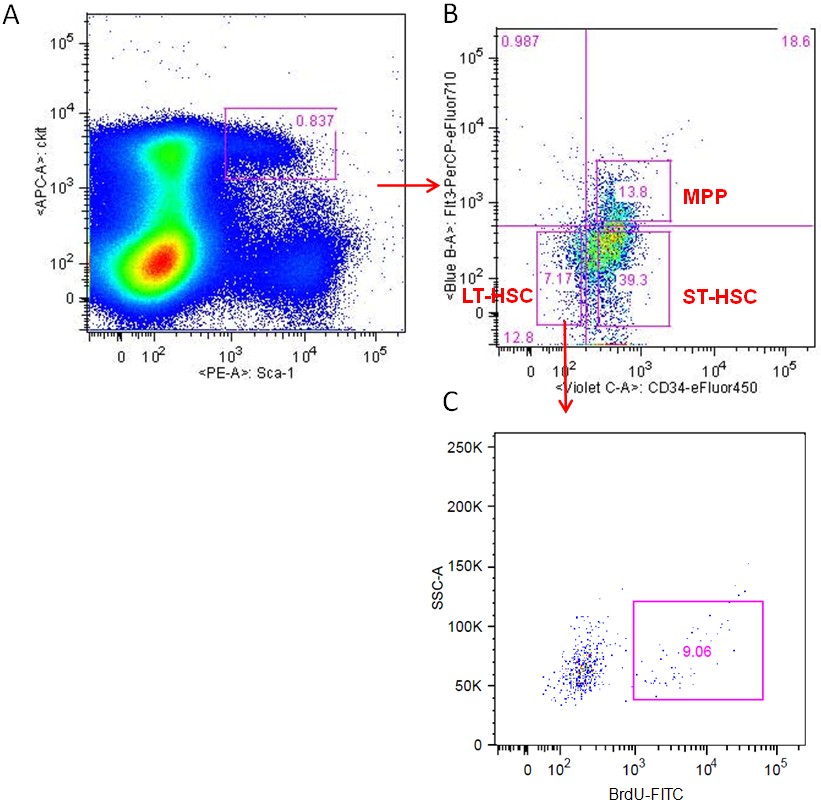
Figure 1. Gating strategy to analyze BrdU incorporation in LT-HSCs. A. BM Lin- cells were labeled with PE-Scal-I and APC-c-Kit antibodies and analyzed by flow cytometry. B. Lin-/Scal-I+/c-Kit+ (LSK) cells were gated as showed in A and the LSK cells were further analyzed with eFluor 450-CD34 and PerCP-eFluor 710-CD135 staining. Long-term HSCs (LT-HSCs, shown as Lin-Sca-1+c-Kit+CD34-CD135-), Short-term HSCs (ST-HSCs, shown as Lin-Sca-1+c-Kit+CD34+CD135-) and multipotent progenitors (MPPs, shown as Lin-Sca-1+c-Kit+CD34+CD135+) were separated as indicated. C. BrdU incorporation was further analyzed for each cell population. Data shown are LT-HSCs population analyzed for BrdU staining.
Recipes
- Buffer A
DPBS, pH 7.2 supplemented with 0.5% BSA and 2 mM EDTA
- Red blood cell lysis buffer
155 mM potassium bicarbonate
10 mM Ammonium chloride
0.1 mM of EDTA, pH = 7.4
- Staining buffer
DPBS, pH 7.2 supplememted with 0.5% BSA and 0.09% sodium azide
Acknowledgments
This protocol is adapted from An et al. (2013).
References
- An, N., Lin, Y. W., Mahajan, S., Kellner, J. N., Wang, Y., Li, Z., Kraft, A. S. and Kang, Y. (2013). Pim1 serine/threonine kinase regulates the number and functions of murine hematopoietic stem cells. Stem Cells 31(6): 1202-1212.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
An, N. and Kang, Y. (2013). In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells. Bio-protocol 3(21): e960. DOI: 10.21769/BioProtoc.960.
Category
Stem Cell > Adult stem cell > Hematopoietic stem cell
Cell Biology > Cell viability > Cell proliferation
Molecular Biology > DNA > DNA labeling
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link