- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Pancreatic Acinar Cell 3-Dimensional Culture
Published: Vol 3, Iss 19, Oct 5, 2013 DOI: 10.21769/BioProtoc.930 Views: 15108
Reviewed by: Lin FangFanglian HeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation of Murine Alveolar Type II Epithelial Cells
Fan Sun [...] Zhaoxia Qu
May 20, 2017 13109 Views

Soft Agar Colony Formation Assay as a Hallmark of Carcinogenesis
Feng Du [...] Daiming Fan
Jun 20, 2017 30552 Views

A Fast and Reliable Method to Generate Pure, Single Cell-derived Clones of Mammalian Cells
Zhe Han [...] Varun Kumar
Aug 20, 2022 4099 Views
Abstract
Normal pancreatic acinar cells are difficult to maintain on traditional plastic culture surfaces due to their physical properties of housing large quantities of digestive enzymes and the formation of intercellular tight junctions and gap junctions (Apte and Wilson 2005; Rukstalis et al., 2003). However, placing primary acinar cells within a 3-dimensional matrix (3D-culture) maintains the cells for sufficient time so that they can be monitored for physiological changes to different stimuli. We have used a modified collagen 3D-culture system that has been adapted from Means et al. (2005) to model the very early events associated with pancreatic cancer development. In this model, KrasG12D-expressing pancreatic acinar cells, or wildtype acinar cells treated with EGFR-dependent growth factors (i.e., TGFα), convert to ductal cysts that mimic the acinar-to-ductal metaplasia (ADM) stage that precedes formation of Pancreatic Intraepithelial Neoplasia (PanIN) and Pancreatic Ductal Adenocarcinoma (PDAC) (Means et al., 2005; Shi et al., 2013).
Keywords: Tissue cultureMaterials and Reagents
- Rat Tail Collagen I (Life Technologies, Invitrogen™, catalog number: A10483 )
- Fetal Bovine Serum (FBS)
- Pen-Strep
- Hanks Balanced Salt Solution (HBSS) (Life Technologies, Gibco®, catalog number: 14065 )
- E.Z.N.Z Total RNA Kit I (Omega Bio-Tek, catalog number: R6834-01 )
- BME-phenol blue
- Xylene
- 10x RPMI 1640 medium (Life Technologies, Gibco®, catalog number: 31800-022 ) (see Recipes)
- 4.2% NaHCO3 (see Recipes)
- Collagenase P (Roche, catalog number: 11213857001 ) (see Recipes)
- 100x Soybean Trypsin Inhibitor (STI) (Sigma-Aldrich, catalog number: T6522 ) (see Recipes)
- 2,000x Dexamethasone (Dex) (Sigma-Aldrich, catalog number: D4902 ) (see Recipes)
- 3D-culture medium (see Recipes)
Equipment
- Centrifuge (Beckman Coulter, model: GS-15R or equivalent, rotor S4180)
- Standard 5% CO2 tissue culture incubator
- 24-well tissue culture plates
- 37 °C shaker
- 6 cm culture dish
- Dissection scissors
- 15 ml plastic screw cap tube
- Laminar flow hood
- Rocking platform
- Tissue processor
- Polypropylene Mesh (105 μm & 500 μm sizes) (Spectra/Mesh) (Fisher Scientific, catalog number: 146436 and 146418 )
Note: Cut meshes into 10 x 10 cm squares and sterilize by autoclaving.
- Homogenizer spin column (Omega Bio-Tek, catalog number: HCR003 )
- Tissue-Teck Biopsy Uni-cassette (Sakura, catalog number: 4087)
- Falcon tube (15 ml and 50 ml)
- Nanodrop spectrophotometer
Procedure
The following procedures for one mouse pancreas
Notes:
- All procedures are performed in a laminar flow hood under sterile conditions.
- For each pancreas prepare 30 ml Cold HBSS + 5% FBS and 20 ml HBSS + 30% FBS.
- Prepare 24-well tissue culture plates (you will need 250 μl collagen mix per well)
- On ice, mix rat tail collagen in a ratio of 0.9 ml collagen: 0.1 ml 10x RPMI 1640 medium. Add 80-100 μl 4.2% NaHCO3 per 1 ml mixture (usually a light pink/yellowish color will be achieved).
Note: The volume of NaHCO3 should be tested and adjusted for each batch of collagen.
- Place each 24-well plate on ice and pipette 250 μl of collagen gel mixture into each well, assuring that the gel covers the bottom of each well. Repeat so as to have a separate plate for each day of harvest. Cover and place in a 37 °C, 5% CO2 incubator for at least 1 h to solidify.
- On ice, mix rat tail collagen in a ratio of 0.9 ml collagen: 0.1 ml 10x RPMI 1640 medium. Add 80-100 μl 4.2% NaHCO3 per 1 ml mixture (usually a light pink/yellowish color will be achieved).
- Isolate cells
- Sacrifice each mouse following institutional IACUC protocols.
- Put the mouse on a dissection plate in a supine position, spray with 70% EtOH and open the abdominal cavity to expose the pancreas.
- Resect the pancreas and place into ~20 ml of HBSS in a 6 cm culture dish on ice. Swirl to wash, and decant liquid.
- Add 5 ml cold HBSS and mince the pancreas quickly with dissection scissors.
- Transfer the pancreas material into a 15 ml plastic screw cap tube. Add 100 μl 10 mg/ml collagenase P to the tissue suspension and mix gently.
- Wrap Parafilm around the cap and shake at 225 rpm in a 37 °C shaker for 15-20 min, until most of the tissue clumps are gone and the suspension look cloudy (examine by eye every 5 min to prevent over- or under-digestion).
- Add 5 ml cold HBSS + 5% FBS and centrifuge 2,000 rpm, 2 min at 4 °C. Aspirate supernatant.
- Resupsend the pellet 3 times in 5 ml cold HBSS + 5% FBS, spinning at 1,500 rpm, 2 min between rinses.
- After the final wash, resuspend the pellet in 5 ml HBSS + 5% FBS.
- With sterilized scissors, cut the tip of the1,000 μl pipette tip to make the opening wider and then gently pipet the cell suspension through a sterile 500 μm mesh.
- Pipet an additional 5 ml HBSS + 5% FBS to wash all remaining cells through the mesh.
- Repeat by pipetting the cell suspension through a 105 μm mesh.
- Slowly pipet the cell suspension on top of a 50 ml tube containing 20 ml HBSS + 30% FBS.
- Centrifuge 1,000 rpm, 2 min, 4 °C to pellet individual acini clusters.
- Aspirate supernatant.
- Sacrifice each mouse following institutional IACUC protocols.
- Plating cells
- Resuspend the cell pellet in 8-10 ml (volume adjusted according to the size of pancreas) in 3D-culture medium.
- Mix the collagen as above step A1.
- Mix equal parts of the cell suspension and collagen mix.
- Immediately plate the cell suspension in the collagen-coated wells, 0.5 ml/well (see Figure 1).
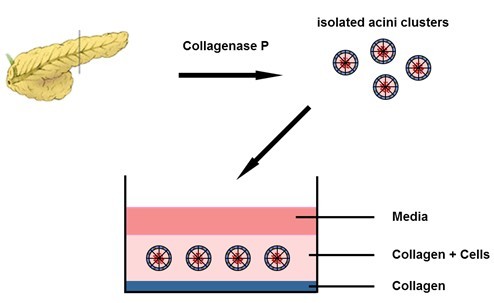
Figure 1. 3D-culture setup
- Allow the collagen-cell mixture to solidify ~1 h at 37 °C, 5% CO2, and then add 1 ml warm 3D-culture medium (with or without growth factors inhibitors).
- Change the medium on days 1 and 3. For KrasG12D-expressing acinar cells, over 90% of ductal cysts should form by day 5 (see Figure 2). Similarly, wild-type acinar cells provided 50 ng/ml TGFα following plating will form ductal cysts by day 5, with a conversion rate of about 70%-80% (see Figure 2) (Shi et al., 2013).
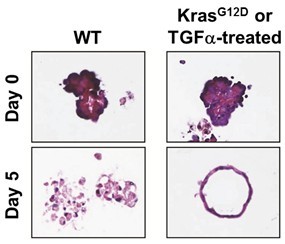
Figure 2. Pancreatic acinar cells in 3D-culture
- Resuspend the cell pellet in 8-10 ml (volume adjusted according to the size of pancreas) in 3D-culture medium.
- RNA/protein prep from acinar cell 3D-culture
- Dilute collagenase P (10 mg/ml) 1:50 in 1x HBSS (room temperature). Prepare 4 ml solution in a 15 ml falcon tube for each sample.
- Wash cells/collagen disc in culture well once with 1x HBSS. Transfer collagen disc to a 15 ml falcon tube with a spatula. Pool 3 or 4 wells together to obtain enough cells.
- Digest collagen for ~ 30 min in a 37 °C shaker, 250 rpm. Check every 10 or 15 min.
- After all of the collagen is digested, pellet the cells at 2,000 rpm for 2 min at 4 °C.
- Remove the supernatant, resuspend cells with 1 ml cold HBSS and transfer to a 1.5 ml centrifuge tube.
- Centrifuge at 5,000 rpm for 2 min at 4 °C. Carefully remove the supernatant. Loosen the pellet by flicking the bottom of the tube with your finger tips.
- OPTION 1: RNA Prep. Follow the E.Z.N.A. Total RNA Mini Kit protocol. Homogenize using a homogenizer column. Resuspend the final RNA in 40 μl ddH2O. Measure the RNA concentration using a nanodrop spectrophotometer. Store at -20 °C.
- OPTION 2: Protein Prep. Prepare 100 μl 4x Sample Buffer (BME-phenol blue) + 1:50 protease inhibitor cocktail + 1:100 phosphatase inhibitor cocktail 1 + 1:100 phosphatase inhibitor cocktail 2 + 1:200 Na3VO4 for each sample. Lyse the cells by pipetting and sonicate 15 seconds. Store at -20 °C.
- Dilute collagenase P (10 mg/ml) 1:50 in 1x HBSS (room temperature). Prepare 4 ml solution in a 15 ml falcon tube for each sample.
- Collagen disc fixation
- Fix the collagen discs in the wells with 10% neutral buffered formalin for 30 min at room temperature, and then separate the discs from the plate wall using a yellow 200 μl pipette tip and transfer the discs into scintillation vials containing 10% neutral buffered formalin. Fix on rocker at RT overnight.
- Transfer the fixed collagen discs from the scintillation vials into standard biopsy cassettes.
- Gradually dehydrate the collagen discs by incubating in 70%, 90%, and 100% EtOH and then in Xylenes in a glass container at room temperature, 2 times of 1 h incubation for each solution on a rocking platform.
- After Xylene clearing, transfer the cassettes into the paraffin tank of the tissue processor. Let the collagen discs go through the last two steps of paraffin incubation (1 h each at 65 °C) and then keep them in paraffin tank until it is time for making paraffin blocks.
- IMPORTANT: Keep cassettes horizontal or the gels will "flow" into the corners and will be difficult to embed.
- Collagen discs can be processed through an automatic tissue processor. However, the freshness (cleanliness) of the solutions inside the processor greatly affects the outcome (the collagen discs will be hard and shrunken when solutions in the processor become old). Manual processing with small volumes of fresh solutions will keep the collagen discs in their original shape after processing.
- IMPORTANT: Keep cassettes horizontal or the gels will "flow" into the corners and will be difficult to embed.
- Fix the collagen discs in the wells with 10% neutral buffered formalin for 30 min at room temperature, and then separate the discs from the plate wall using a yellow 200 μl pipette tip and transfer the discs into scintillation vials containing 10% neutral buffered formalin. Fix on rocker at RT overnight.
Recipes
- 10x RPMI 1640 medium
Dissolve 1 package of RPMI 1640 powder in 100 ml ddH2O
Add 2 g NaHCO3, pH to 7.2
Filter sterilize
Store at 4 °C
Make fresh solution every 2 months
- 4.2% NaHCO3
Dissolve 4.2 g NaHCO3 in 100 ml ddH2O
Filter sterilize
Store at 4 °C
- Collagenase P
Dissolve in 10 ml ddH2O to make a 10 mg/ml stock
Filter sterilize, aliquot and store at -80 °C
Thaw a new vial for each acinar prep
- 100x Soybean Trypsin Inhibitor (STI)
Dissolve in ddH2O to make a 10 mg/ml stock
Filter sterilize, aliquot and store at -20 °C
- 2,000x Dexamethasone (Dex)
Dissolve 25 mg in 12.5 ml of 100% EtOH to generate a 2 mg/ml stock
Aliquot and store at -20 °C
- 3D-culture medium
RPMI 1640
1% FBS
1% Pen-Strep
0.1 mg/ml STI
1 μg/ml Dex
Acknowledgments
This protocol was adapted from Shi et al. (2013).
References
- Apte, M. and Wilson, J. (2005). The importance of keeping in touch: regulation of cell-cell contact in the exocrine pancreas. Gut 54(10): 1358-1359.
- Means, A. L., Meszoely, I. M., Suzuki, K., Miyamoto, Y., Rustgi, A. K., Coffey, R. J., Wright, C. V., Stoffers, D. A. and Leach, S. D. (2005). Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132(16): 3767-3776.
- Rukstalis, J. M., Kowalik, A., Zhu, L., Lidington, D., Pin, C. L. and Konieczny, S. F. (2003). Exocrine specific expression of Connexin32 is dependent on the basic helix-loop-helix transcription factor Mist1. J Cell Sci 116(16): 3315-3325.
- Shi, G., DiRenzo, D., Qu, C., Barney, D., Miley, D. and Konieczny, S. (2013). Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia. Oncogene 32(15): 1950-1958.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Qu, C. and Konieczny, S. F. (2013). Pancreatic Acinar Cell 3-Dimensional Culture. Bio-protocol 3(19): e930. DOI: 10.21769/BioProtoc.930.
Category
Cancer Biology > General technique > Cell biology assays > Cell isolation and culture
Cell Biology > Cell isolation and culture > 3D cell culture
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










