- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Labeling of Precursor Granule Cells in the Cerebellum by ex vivo Electroporation
Published: Vol 3, Iss 12, Jun 20, 2013 DOI: 10.21769/BioProtoc.778 Views: 15453

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Parkinson’s Disease-relevant Mitochondrial and Neuronal Morphology High-throughput Screening Assay in LUHMES Cells
Tom Leah [...] Heather Mortiboys
Jan 5, 2021 5891 Views

Spatial Centrosome Proteomic Profiling of Human iPSC-derived Neural Cells
Fatma Uzbas and Adam C. O’Neill
Sep 5, 2023 2542 Views

Primary Neuronal Culture and Transient Transfection
Shun-Cheng Tseng [...] Eric Hwang
Jan 20, 2025 2702 Views
Abstract
This protocol will be useful to introduce the genes of interest into the cerebellar granule cells at early stages of development. Since the granule cell precursors are localized in the external granule layer before migration, DNA plasmids can be specifically incorporated into the granule cells by injecting DNA solution into the cerebellar fissures followed by application of electric pulses. This technique can be performed prior to the preparation of either dissociated or organotypic culture, which can be used to study the molecular mechanisms of cell migration, axon elongation and synapstogenesis during development.
Keywords: ElectroporationMaterials and Reagents
- Plasmid Maxi Kit (QIAGEN)
- 10x HEPES buffered saline (HBS)
- Fast green FCF (Sigma-Aldrich, catalog number: F7252 )
- 1x Phosphate buffered saline (PBS)
- 10x HBS (see Recipes)
Equipment
- Capillary glass (Harvard Apparatus, catalog number: 30-0066 )
- Micropipette puller P-97/ IVF (Sutter Instrument)
- Aspirator Tube Assembly (Drummond, catalog number: 2-000-000 )
- Square pulse electroporator and a foot switch (Nepagene, catalog number: CUY21 )
- Platinum plate tweezers-type electrode (Protech International, catalog number: CUY650-P5 )
- Dissecting microscope
- Dissecting tools: Forceps and some fine scissors
- 10 cm-petri dish
Procedure
A. Preparation of micropipettes for DNA injection
- Pull the capillary glass using the puller with a single pull. Adjust the puller setting to make the capillary taper approximately 10-20 mm. When using P-97, start with the following setting: Heat equal of the Ramp value, Pull 0, Vel 40 and Time 200.
- Cut out 1/3 – 1/2 of the tips of the pulled pipettes using the forceps.
B. DNA preparation
- Prepare plasmids using the Plasmid Maxi Kit and elute DNA with distilled water. The concentration of the DNA should be 4-5 μg/μl for storage.
- For injection, the final concentration of DNA should be 2 μg/μl. Add 1/10 volume of 10x HBS, 1/10 volume 1% fast green (final 0.1%) and water to the DNA solution to adjust the concentration.
C. Electroporation
- Attach a micropipette to an aspirator tube assembly and draw 10 μl of DNA -solution into the pipette (Figure 1).
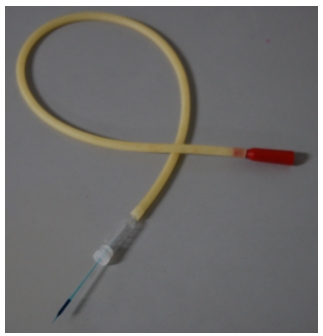
Figure 1. An aspirator tube and a glass capillary
- Remove a whole-brain from a mouse (postnatal days 7-9) and put it into an ice-cold PBS in a 10-cm petri dish.
- Remove the forebrain and the brain stem using the forceps. If the cerebellum is planned to be used for organotypic culture, the meninges should not be removed in order to keep the cellular layers intact. If the cerebellum is to be further processed for dissociated culture, the meninges should be removed to avoid glial cell overgrowth.
- Observe the cerebellum under a dissecting microscope. Inject the DNA solution (2 -3 μl/fissure) into the cerebellar fissures by mouth pipetting (Figure 2). Inject into at least 2 or 3 fissures, starting from those in the vermis. If high transfection efficiency is required, inject into as many fissures as possible.
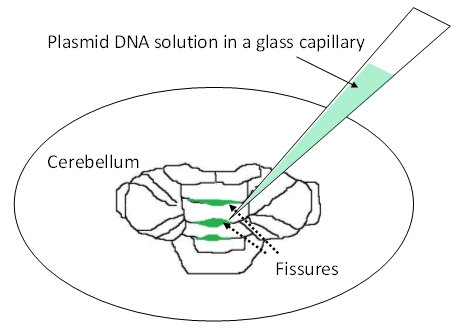
Figure 2. Injection of plasmid DNA solution into cerebellar fissures
- Place the forceps-type electrodes so that cerebellum is in between the two electrode planes. The two planes should be positioned parallel to the fissures of the cerebellar vermis. They should be held about 3-5 mm apart from the cerebellum surface (Figure 3).
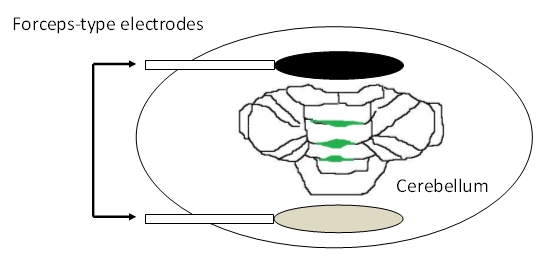
Figure 3. Placement of electrodes
- Apply electric pulses; 99.9 V, ON 50 msec, OFF 450 msec, 5 pulses. Adjust the voltage value depending on the outcome. When the transfection efficiency is too low, the voltage should be increased up 120 V. When the transfection efficacy is too high or when the number of dying cells is too high, the voltage setting should be decreased.
- The cerebellum can be further processed for either organotypic slice or dissociated cultures (please see reference 1 for the images showing the labeled granule cells).
Recipes
- 10x HBS
10x conc. (mM)
g/100 ml H2O
NaCl
1,400
8.2
Na2HPO4.2H2O
15
0.3
HEPES
500
11.9
Acknowledgments
This protocol is adapted from Yang et al. (2004) and Ito-Ishida et al. (2012).
References
- Ito-Ishida, A., Miyazaki, T., Miura, E., Matsuda, K., Watanabe, M., Yuzaki, M. and Okabe, S. (2012). Presynaptically released Cbln1 induces dynamic axonal structural changes by interacting with GluD2 during cerebellar synapse formation. Neuron 76(3): 549-564.
- Yang, Z. J., Appleby, V. J., Coyle, B., Chan, W. I., Tahmaseb, M., Wigmore, P. M. and Scotting, P. J. (2004). Novel strategy to study gene expression and function in developing cerebellar granule cells. J Neurosci Methods 132(2): 149-160.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ito-Ishida, A. (2013). Labeling of Precursor Granule Cells in the Cerebellum by ex vivo Electroporation. Bio-protocol 3(12): e778. DOI: 10.21769/BioProtoc.778.
Category
Developmental Biology > Cell growth and fate > Neuron
Neuroscience > Development > Neuron
Molecular Biology > DNA > Transformation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








