- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessing the Presence of Hematopoietic Stem and Progenitor Cells in Mouse Spleen
Published: Vol 12, Iss 11, Jun 5, 2022 DOI: 10.21769/BioProtoc.4438 Views: 3766
Reviewed by: Chiara AmbrogioDhruv Rajanikant PatelKuo-Ching "KC" MeiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2542 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views
Abstract
Transplantation of hematopoietic material into recipient mice is an assay routinely used to determine the presence and function of hematopoietic stem and progenitor cells (HSPCs) in vivo. The principle of the method is to transplant donor cells being tested for HSPCs into a recipient mouse following bone marrow ablation and testing for reconstitution of hematopoiesis. Congenic mouse strains where donor and recipient differ by a distinct cell surface antigen (commonly CD45.1 versus CD45.2) are used to distinguish between cells derived from the donor and any residual recipient cells. Typically, the transplantation is performed using bone marrow cells, which are enriched for HSPCs. Here, we describe an analogous procedure using hematopoietic material from spleen, allowing detection of functional progenitors and/or stem cells in the spleen that can occur under certain pathologies. Key to the success of this procedure is the prior removal of mature T cells from the donor sample, to minimize graft versus host reactions. As such, this protocol is highly analogous to standard bone marrow transplant procedures, differing mainly only in the source of stem cells (spleen rather than bone marrow) and the recommendation for T cell depletion to avoid potential immune incompatibilities.
Graphical abstract:
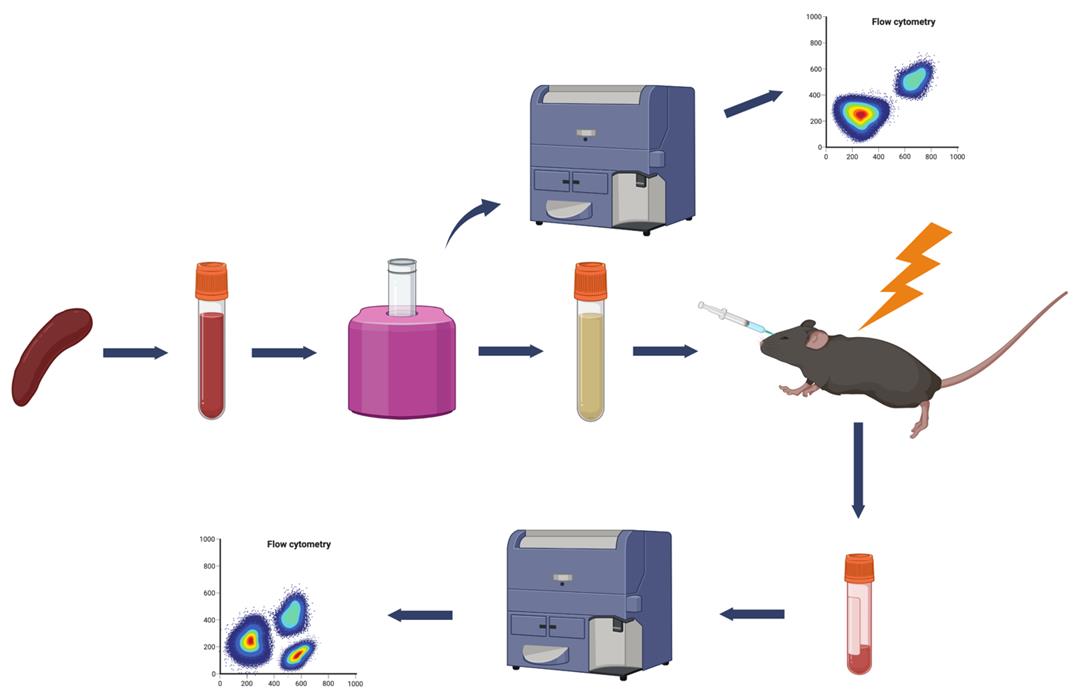
Schematic overview for assessment of stem cells in spleen by transplantation. Single cell suspensions from spleens are depleted of potentially pathogenic mature T lymphocytes by magnetic bead immunoselection using biotinylated antibodies against CD4 and CD8, followed by streptavidin magnetic beads, which are subsequently removed by using a magnet (MojoSort, Biolegend). Successful T cell depletion is then evaluated by Fluorescence Activated Cell Sorting (FACS). T-cell depleted cell suspension is injected intravenously through the retro-orbital sinus into lethally irradiated recipients. Recipients are analyzed for successful engraftment by FACS analysis for the presence of donor-derived mature hematopoietic lineages in the peripheral blood. A second serial transplantation can be used to document the presence of long-term reconstituting stem cells in the periphery of the original donor mice.
Background
The hematopoietic system of mice can be regenerated from hematopoietic stem and progenitor cells (HSPCs). In adult mice, HSPCs largely reside in the bone marrow, where they are maintained in a largely quiescent state. However, during some inflammatory pathologies, HSPCs become mobilized and can colonize various peripheral lymphoid organs, such as spleen. Definitive assessment of the presence and function of HSPCs is accomplished by observing their ability to reconstitute the full hematopoietic system of an irradiated recipient mouse (so the HSPCs of the recipient mouse are depleted) following transplantation. Committed progenitors will give rise to only a subset of hematopoietic lineages in this assay, while true hematopoietic stem cells (HSCs) will be capable of reconstituting all mature blood lineages. Stem cells with long-term reconstitution ability can be distinguished from short-term stem cells by secondary transplantation. While short-term HSCs are capable of reconstituting hematopoiesis in an initial transplant, only long-term HSCs will give rise to pluripotent stem cells that are capable of reconstituting a secondary recipient.
The present protocol, adapted from a recent publication (Marié et al., 2021), describes a method for assessing the presence of HSPCs in mouse spleens by serial engraftment in recipient mice whose hematopoietic system has been ablated by x-ray irradiation. Antigenic differences between donor and recipients allow the detection of donor cells following engraftment by a simple flow cytometry analysis. Flow cytometry is also used to assess the presence of major hematopoietic lineages derived from donor cells through analysis of characteristic cell surface markers. Following primary reconstitution, the presence of short-term versus long-term HSPCs can be assessed by conducting a secondary engraftment and observing the reconstitution of hematopoietic lineages in the secondary recipients. Moreover, the ability of the donor HSPCs to home to the bone marrow versus secondary lymphoid organs can be assessed by performing secondary reconstitutions with cells derived from either bone marrow or spleen. In all transplantations involving cells from peripheral organs, it is essential to deplete mature T lymphocytes to minimize graft versus host reactions. A potential limitation of this technique is that its sensitivity has not yet been strictly defined. At present, the minimum threshold of stem cell abundance in the spleen capable of triggering a successful transplant needs to be defined. This limitation could be addressed by including serial dilution studies.
Materials and Reagents
35 mm culture dish (Falcon, catalog number: 3001)
3 mL syringe (BD Biosciences, catalog number: 305270)
1 mL syringes (BD, catalog number: 320933)
15 mL conical tubes (BD Biosciences, Falcon®, catalog number: 352196)
50 mL conical tubes (BD Biosciences, Falcon®, catalog number: 430829)
70 μM nylon strainers (BD Biosciences, Falcon®, catalog number: 352350)
Eppendorf tubes 1.5 mL (Axygen®, catalog number: MCT-175-C)
FACS tubes 5 mL (BD Biosciences, catalog number: 352054)
C57BL6 (CD45.2) donor mice (bred in our laboratory by mating a male and a female both CD45.2)
C57BL6 (CD45.1) recipient mice (bred in our laboratory by mating a male and a female both CD45.1)
Antibiotic suspension of Sulfamethoxazole (40 mg/mL) and Trimethoprim (8 mg/mL) in 0.03% ethanol, Ani Pharmaceuticals
Phosphate Buffered Saline (PBS) (without Ca2+ and Mg2+) (Sigma-Aldrich, catalog number: D8537)
BV711 mouse anti-mouse CD45.1 (cloneA20) (Biolegend, catalog number: 110739) 0.2 mg/mL
BV605 mouse anti-mouse CD45.2 (clone104) (Biolegend, catalog number: 109841) 0.2 mg/mL
Biotin rat anti-mouse CD4 (clone RM4-5) (Biolegend, catalog number:100508) 0.5 mg/mL
Biotin rat anti-mouse CD8a (clone 53-6.7) (Biolegend, catalog number: 100704) 0.5 mg/mL
PE Cy5 rat anti-mouse CD4 (clone RM4-5) (Biolegend, catalog number: 100514) 0.2 mg/mL
APC rat anti-mouse CD8a (clone 53-6.7) (Biolegend, catalog number: 100712) 0.2 mg/mL
PE Cy7 rat anti-mouse CD11b (M1/70) (Biolegend, catalog number: 101215) 0.2 mg/mL
APC-eFluor 780 rat anti-mouse B220 (RA3-6B2) (eBiosciences, catalog number: 47-0452-82) 0.2 mg/mL
Fetal Bovine Serum (FBS) (Gibco, catalog number: 10437-028)
Red Blood Cell (RBC) lysing buffer (BD PharmLyseTM lysing buffer, BD Biosciences, catalog number: 555899)
MojoSortTM Buffer (Biolegend, catalog number: 480017)
DMEM medium (Corning, Cellgro, catalog number: 10-013-CV)
MojoSortTM Streptavidin nanobeads (Biolegend, catalog number: 480016)
Needles (27G1/2) (BD Biosciences, catalog number: 305109)
Goldenrod animal lancet 5mm (Medipoint)
BD EDTA-coated Microtainer (BD Biosciences, catalog number: 365974)
FACS buffer (see Recipes)
Equipment
Forceps and sharp scissors
Inverted microscope
Ice bucket
Hemacytometer (Sigma-Aldrich, catalog number: Z359629-1EA)
Centrifuge (low-speed for 15 and 50 mL tubes)
Centrifuge for microcentrifuge tubes (1.5 mL)
MojoSort Magnet (Biolegend, catalog number: 480019), cooled at 4°C the day before
Mice Irradiation machine (Faxitron MR350 X-ray irradiator)
BD LSR II flow cytometer
Software
FACSDiva software
FlowJo software
Procedure
Part I: Transplantation of spleen cells
The following protocol has been adapted from the more traditional bone marrow transplantation protocol (Duran-Struuck and Dysko, 2009). Bone marrow transplantation is routinely used to assess the presence and efficiency of progenitors/stem cells in a given bone marrow sample. While normally virtually absent in the spleen, HSPCs may accumulate in the spleen during some pathological processes, for example, during inflammation, splenomegaly, or extramedullary hematopoiesis. Our protocol aims at detecting the presence of functional progenitors and/or stem cells in the spleen.
It is important to note that spleens contain more reactive T cells than bone marrow, which can lead to adverse effects during engraftment, such as graft versus host disease (Baker et al., 1996). To avoid such potential problems, mature T cells are depleted before transplantation.
Irradiation of host mice
The hematopoietic system of recipient mice is ablated by lethal irradiation applied in two fractional doses separated by 4 h.
Irradiate C57BL6 (CD45.1) 8- to 12-week-old host mice, 20 to 25 g, sex irrelevant (10 mice per transplantation group) with a first dose of 4.5 Gy total body irradiation (TBI), (Faxitron MR350 X-ray irradiator using SnCuAl filter) using a mouse irradiation pie cage (Figure 1). Following irradiation, supplement the drinking water with 1:200 dilution of antibiotic suspension of Sulfamethoxazole and Trimethoprim (Materials and Reagents #11).
Irradiate host mice with a second dose of 4.5 Gy 4 h later, just before transplantation. In the meantime, proceed to the preparation of donor material.

Figure 1. Mouse irradiation pie cage. Mice are placed in an irradiation pie cage prior to exposure to x-ray irradiation.Preparation of donor material
The whole procedure has to be carried out under sterile conditionsSacrifice C57BL6 donor mice (CD45.2) by CO2 euthanasia. Collect the spleen (Figure 2A) and place in PBS on ice. Trim away any remaining connective tissue or fat from the spleen.
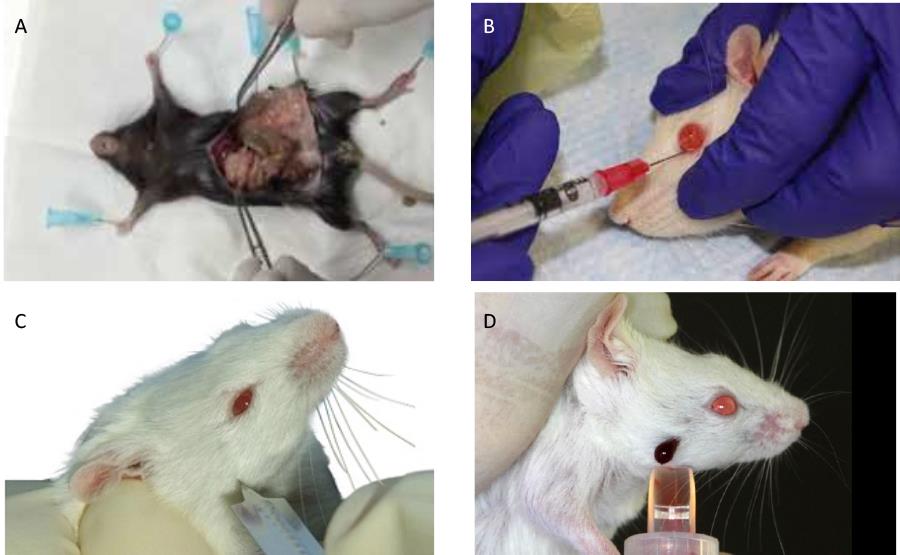
Figure 2. Mouse bleeding, injection, and spleen recovery procedures. (A) Illustration of post-mortem spleen collection. (B) Fluid injection into the retro-orbital sinus. (C) Puncturing the submandibular vein with a lance. (D) Blood collection from a pierced submandibular vein.Transfer the spleen into a sterile 35 mm culture dish containing 5 mL of FACS buffer (see Recipes below) or DMEM medium containing 2% FBS.
Remove the plunger from a 3 mL syringe. Use the flat rubber end of the plunger to crush the spleen by using gentle circular motions. This will disrupt the pulp and release the splenocytes.
Pass the 5 mL of cell suspension through a 70 μm nylon strainer fitted on top of a 50 mL conical tube to obtain a uniform single-cell suspension. Gently help the suspension pass through the strainer by pressing in a circular manner with the syringe plunger. A video presentation of this method can be seen at https://www.stemcell.com/prepare-single-cell-suspension-from-mouse-spleen.html.
Wash the 35 mm culture dish with 2 mL of FACS buffer or DMEM medium containing 2% FBS
and pass through the strainer. Repeat this step twice.
Centrifuge the tube at 300 × g for 10 min at 4°C.
Discard the supernatant and flick the tube to loosen the pellet. Resuspend the pellet in a volume of 200 μL to 1 mL of medium containing 3% FBS, depending on the spleen size, and store on ice.
Count the cells after diluting an aliquot of the cell suspension 1:100 with PBS. Count all nine squares of the hemacytometer under an inverted light microscope. One normal spleen (approximately 100 mg) typically produces around 100 million cells. We recommend using a live dye such as trypan blue (1:1 v/v) to exclude potential dead cells (Figure 3).
Note: Since the cell suspension has not undergone red blood cell lysis, it is important to make sure that the red blood cells (smaller than the white blood cells) are not counted (Figure 3).
The cell count per mL is the number of cells counted × dilution factor × 104 (the dilution factor being 100 in this case).
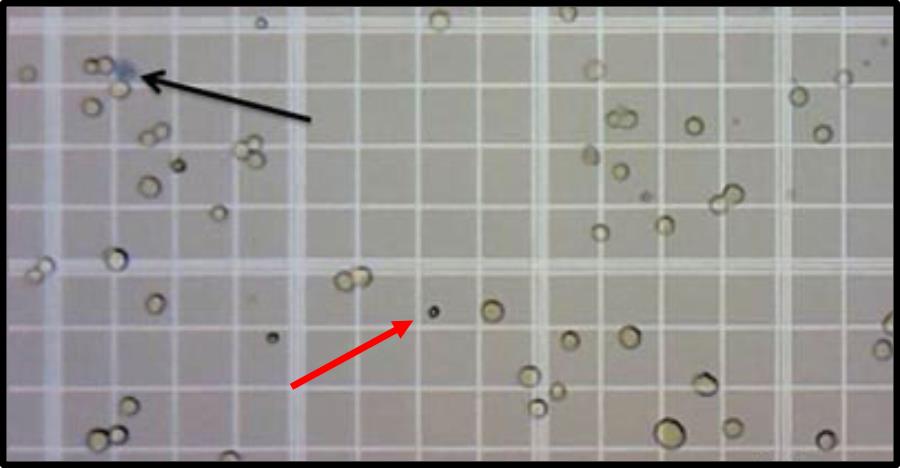
Figure 3. Peripheral blood cell counting with a hematocytometer. Viable leukocytes are counted on a hematocytometer grid. Red arrow points to a red blood cell, and black arrow points to a trypan blue-stained dead cell, neither of which are included in the leukocyte count.
T cell depletionTransfer 108 cells resuspended in 500 μL of MojoSort Buffer into a 5 mL (12 × 75 mm) polystyrene tube. Add 5 μL each of biotin-anti CD4 and biotin-anti CD8a to the tube, mix well, and incubate on ice for 15 min.
Note: Keep a small aliquot (106 cells) of starting material prior to depletion (step B7) on ice, to be used from step 20 onward, to assess the extent of the depletion after the procedure.
Wash the cells by adding 3.5 mL of MojoSort buffer and centrifuge at 300 × g for 5 min at 4°C.
Discard the supernatant and resuspend in 500 μL of MojoSort Buffer.
Resuspend the Streptavidin Nanobeads by vortexing, at maximum speed, five touches. Add 50 μL of beads to the cell suspension, mix well, and incubate for 15 min on ice.
Wash the cells by adding 3 mL of MojoSort Buffer; Centrifuge at 300 × g for 5 min at 4°C, discard the supernatant and resuspend in 3 mL of MojoSort Buffer.
Place the tube in a cold MojoSort magnet (see Equipment) for 5 min.
Collect the cells by pouring out the liquid into a 15 mL conical tube. These are your cells of interest, depleted of T cells that are retained on the magnet.
To increase yield if needed, add 3 mL of MojoSort Buffer to the beads, repeat steps 16 and 17, and pool the flow-through fractions.
Count the cells obtained after depletion as indicated in steps B8 to B10.
Assessment of T cell depletion by FACSTake an aliquot containing 100,000 to 500,000 cells from the sample set aside before T cell depletion (step B7) and from the T cell-depleted sample (step B18). Set up three additional tubes, one for unstained control and two for single color controls containing each 50,000 to 100,000 cells per tube.
Centrifuge the pre- and post- depletion samples, as well as the two single color controls at 200 × g for 5 min at 4°C. Resuspend the pre- and post- depletion samples in 50 μL of staining solution containing PeCy5 anti-mouse CD4 (diluted 1:250) and APC anti-mouse CD8a (diluted 1:250) in FACS buffer.
Resuspend one single color control in 50 μL of PeCy5 anti-mouse CD4 (diluted 1:250) and the other single color control in 50 μL of APC anti-mouse CD8a (diluted 1:250).
Incubate on ice for 15 to 30 min, protected from light.
Wash all tubes with 300 μL of FACS buffer by centrifugation.
Resuspend in 200 μL of FACS buffer and transfer to FACS tubes by passing through a mesh filter to remove any clumps of cells.
Analyze all tubes on a flow cytometer.
On the BD LSR II software, select 'New Experiment'.
Select for Area, Height, and Width for FSC and SSC; select for Log and Area for the colors Pe-Cy5 and APC.
Create compensation controls and adjust the gating for unstained blood cells (adjust FSC and SSC voltages as necessary).
Adjust each color of single stains in the voltage panel so that the positive peak is at the 104 mark.
Analyze single color controls.
Record the desired voltages after any adjustments for each of the single stains and then calculate compensation controls.
Events are now ready to be recorded. Set up to collect 5,000–10,000 events per sample for each pre- and post-depletion sample.
Note: Typically, the procedure depletes over 95% of CD4 and CD8 T cells. The overall depletion procedure is illustrated in the following video: https://www.youtube.com/watch?v=cH6rlIFNVp0.
Transplantation into irradiated mice
Centrifuge the cell suspension of T cell-depleted splenocytes for 5 min at 200 × g at 4°C, remove supernatant, and resuspend in sterile PBS containing 2% of FBS at a cell concentration of 2 × 106 per 100 μL. Keep on ice.
Set up an isoflurane anesthesia machine.
Place an irradiated mouse within the anesthesia chamber.
Turn on the oxygen to flow at 1–2 L/min and the isoflurane gauge to 2–5%.
The animal should become anesthetized within a few minutes.
Load 100 μL of spleen cell suspension into a 1 mL syringe fitted with a 27 G needle.
When the mouse is anesthetized, remove it from the chamber. Promptly, place the animal on absorbent paper, head tilted sideways. With one hand, retract the fur around the eye to make it protrude a little. With the other hand, insert the needle at a 45° angle in the inner corner of the eye to penetrate the retro-orbital sinus (Yardeni et al., 2011). See Figure 2B.
Slowly and smoothly inject the 100 μL of spleen cell suspension (approx. 5 s) and return the mouse to the cage as soon as it starts waking up.
Repeat the procedure for each irradiated animal to be transplanted.
Following injection, the recipient mice are left to recover for 4–6 weeks. Antibiotics can be removed from drinking water after two weeks. Monitor the viability of the transplanted mice every day. The presence of adequate stem cells in the test sample being evaluated should result in greater than 80% recipient survival.
Remember to have proper irradiation controls. These mice are comparable to the ones used for the transplant (same age and sex) that will be irradiated with the experimental mice, but they will not be transplanted. They should become moribund after 10–14 days, at which point they are euthanized.
Part II: Blood reconstitution analysis
First analysis of total blood donor reconstitution is done between 4–6 weeks following cell transplantation. This analysis aims to determine whether donor cells have engrafted by assessing the presence of CD45.2 cells (from the donor) in the recipient blood.
Obtaining total white blood cells for flow cytometry
Blood collection is performed by puncture of the facial vein that runs from the submandibular vein across the cheek (Regan et al., 2016). See Figure 2C and 2D.
Hold the mouse firmly by the scruff in a vertical position and puncture skin at the rear of the jawbone, at the intersection of two virtual lines, one originating at the external corner of the eye and the other one at the hairless spot close to the mouth, with a Goldenrod animal lancet.
Collect blood into an EDTA-coated BD microtainer; 200 µL is sufficient (approximately four drops).
Keep doing the same for all the recipient mice of the experiment. In addition, bleed a non-transplanted CD45.2 mouse and a non-transplanted CD45.1 mouse to perform the controls for flow cytometry.
Transfer blood samples into 15 mL conical tubes. Perform red blood cell lysis by adding 1mL of BD PharmLyse lysing solution diluted 1:10 in water. Gently vortex each tube and incubate on ice, protected from light, for 15 min.
Centrifuge the tubes at 200 × g for 5 min at 4°C. Carefully aspirate the supernatant without disturbing the pellet (which should appear almost white) and add 2 mL of 1× PBS containing 1% heat-inactivated FBS and 0.1% sodium azide.
Labeling cells for flow cytometry
Centrifuge the tubes at 200 × g for 5 min at 4°C. Carefully aspirate the supernatant without disturbing the pellet and resuspend in 50 µL of staining solution made of a mix of BV711 anti-mouse CD45.1 antibody (diluted 1:200) and BV605 anti-mouse CD45.2 antibody (diluted 1:200) in FACS buffer.
Prepare two samples for single color controls by adding 50 µL of BV711 anti-mouse CD45.1 antibody (diluted 1:200) in the CD45.1 blood sample and 50 µL of BV605 anti-mouse CD45.2 antibody (diluted 1:200) in the CD45.2 blood and retain one sample as unstained.
Incubate on ice for 15 to 30 min, protected from light.
Wash with 300 µL of FACS buffer. Centrifuge the cells, 200 × g, 5 min at 4°C. Aspirate the supernatant.
Resuspend in 200 µL of FACS buffer and transfer to FACS tubes through a mesh filter to remove clumps of cells.
Analyze on Flow cytometer as previously described (steps B27 to B33).
Part III: Long-term engraftment data analysis
This analysis aims at determining whether the engrafted transplanted cells gave rise to all lineages of white blood cells and is performed at least 4 months following transplantation.
Bleed animals and perform red blood cell lysis as previously described (steps A1 to A6).
Stain blood with a staining mix composed of PE-Cy5 anti-mouse CD4 (diluted 1:200), APC anti-mouse CD8a (diluted 1:200), PE-Cy7 anti-mouse CD11b (diluted 1:500), APC-eFluor 780 anti-mouse B220 (diluted 1:200), and BV605 anti-mouse CD45.2 (diluted 1:200) in FACS buffer.
Proceed as detailed in part II-B.
This analysis will determine the percentage of CD4 and CD8 T cells, B cells, and myeloid cells originating from the CD45.2-positive transplanted cells. The presence of all major lineages indicates the presence of HSPCs in the donor material, while the presence of only myeloid cells of donor origin indicates that only committed progenitors were present in the experimental spleen.
To firmly assert that multipotent stem cells were present in the donor spleen, we recommend performing a secondary transplant using the spleen and/or bone marrow of the primary recipients and recapitulating the entire procedure. The presence of blood cell lineages from donor cells in recipients following secondary transplant provides definitive evidence for the presence of totipotent hematopoietic stem cells in the spleens of the primary animals. Depending on the experimental system being studied, the long-term HSCs engrafted during the primary transplant may reside exclusively in the bone marrow or may have also been homed to peripheral organs, which can be evaluated by performing secondary engraftments using either bone marrow or spleen cells.
It will be of interest to apply this protocol to additional secondary lymphoid organs other than spleen. In particular, this technique could be applied to measure the potential presence of hematopoietic stem cells in lymph nodes or other tissues with abundant hematopoietic cells, such as the small and large intestine.
Recipes
FACS buffer
Bovine Serum Albumin (BSA) 0.5% (w/v)
1 mM EDTA
0.05% sodium azide (w/v)
Dissolved in PBS
Filter sterilize using a 0.2 μm filter
Acknowledgments
The protocol was adapted from the previously published paper: Marié et al. (2021). This work was supported in part by National Institutes of Health grants R01AI28900 and Lupus Research Alliance grant 579817 to DEL, and by the Laura and Isaac Perlmutter Comprehensive Cancer Center support grant P30CA016087 from the National Cancer Institute.
Competing interests
The authors declare no competing interests.
Ethics
All work in the development of this protocol was approved by the Institutional Animal Care and Use Committee of NYU Grossman School of Medicine.
References
- Baker, M. B., Altman, N. H., Podack, E. R. and Levy, R. B. (1996). The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J Exp Med 183(6): 2645-2656.
- Duran-Struuck, R. and Dysko, R. C. (2009). Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J Am Assoc Lab Anim Sci 48(1): 11-22.
- Marié, I. J., Brambilla, L., Azzouz, D., Chen, Z., Baracho, G. V., Arnett, A., Li, H. S., Liu, W., Cimmino, L., Chattopadhyay, P., et al. (2021). Tonic interferon restricts pathogenic IL-17-driven inflammatory disease via balancing the microbiome. Elife 10: e68371.
- Regan, R. D., Fenyk-Melody, J. E., Tran, S. M., Chen, G. and Stocking, K. L. (2016). Comparison of Submental Blood Collection with the Retroorbital and Submandibular Methods in Mice (Mus musculus). J Am Assoc Lab Anim Sci 55(5): 570-576.
- Yardeni, T., Eckhaus, M., Morris, H. D., Huizing, M. and Hoogstraten-Miller, S. (2011). Retro-orbital injections in mice. Lab Anim (NY) 40(5): 155-160.
Article Information
Copyright
Marie et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Marie, I. J., Brambilla, L. and Levy, D. E. (2022). Assessing the Presence of Hematopoietic Stem and Progenitor Cells in Mouse Spleen. Bio-protocol 12(11): e4438. DOI: 10.21769/BioProtoc.4438.
- Marié, I. J., Brambilla, L., Azzouz, D., Chen, Z., Baracho, G. V., Arnett, A., Li, H. S., Liu, W., Cimmino, L., Chattopadhyay, P., et al. (2021). Tonic interferon restricts pathogenic IL-17-driven inflammatory disease via balancing the microbiome. Elife 10: e68371.
Category
Immunology > Animal model > Mouse
Stem Cell > Adult stem cell > Hematopoietic stem cell
Cell Biology > Cell Transplantation > Isograft transplantation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









