- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Parasitemia Evaluation in Mice Infected with Schistosoma mansoni
Published: Vol 11, Iss 10, May 20, 2021 DOI: 10.21769/BioProtoc.4017 Views: 4529
Reviewed by: Alexandros AlexandratosEmilie ViennoisAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2516 Views

Assay for Site-Specific Homologous Recombination Activity in Adherent Cells, Suspension Cells, and Tumor Tissues
Yuki Yoshino [...] Natsuko Chiba
Apr 5, 2025 2431 Views

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3976 Views
Abstract
Schistosomiasis is a neglected tropical disease. Its treatment relies on the use of a single drug, praziquantel. Due to treatment limitations, an alternative for schistosomiasis chemotherapy is required; thus, a better understanding of parasite biology and host-parasite interactions is valuable to aid the identification of new anti-Schistosoma drugs. The parasite has a complex life cycle, which results in challenges regarding the evaluation of Schistosoma mansoni development and mammalian infection establishment. Accordingly, this protocol describes methodologies to evaluate: (1) adult worm growth; (2) reproduction; and (3) granuloma formation; and consequently allows more comprehensive knowledge of S. mansoni development in a natural biological system.
Keywords: Schistosoma mansoniBackground
Schistosomiasis is a neglected tropical disease currently endemic in 78 countries (World Health Organization, 2018). Five Schistosoma species can cause parasitosis and are distributed in Asia, Africa, and the Americas. There are two distinct forms of the disease: urogenital, caused by Schistosoma haematobium; and intestinal, caused by Schistosoma intercalatum, Schistosoma mekongi, Schistosoma japonicum, and Schistosoma mansoni (Uberos and Jerez-Calero, 2012; World Health Organization, 2018). Humans are infected following contact with cercariae released from infected aquatic snails.
Here, we focus on the S. mansoni species. After entering a mammalian host, cercariae differentiate into schistosomula (Hitchcock, 1949; de Waard and Vermeulen, 1961; Stirewalt, 1974), which migrate to intrahepatic vessels and develop in adults (Armstrong, 1965; Miller and Wilson, 1980). After mating, the adult worms migrate to the mesenteric veins, where they complete their development and start laying eggs after the 35th day of infection. A female adult worm lays about 300 eggs per day, which reach tissues and the intestinal lumen and are eliminated along with the feces to the external environment (Moore and Sandground, 1956; Gryseels et al., 2006). However, some eggs become trapped in the liver and induce the formation of granulomatous lesions (Espírito Santo et al., 2008; King and Dangerfield-Cha, 2008), which can lead to fibrosis in host tissues and the promotion of injuries and complications that cause anemia, malnutrition, and loss of quality of life, having a high socioeconomic impact (Burke et al., 2009; Olveda et al., 2017).
Schistosomiasis treatment relies on the use of praziquantel, which displays activity against all species; although, immature worms are not affected (Watson, 2009; Thétiot-Laurent et al., 2013). Additionally, there are reports of adult worms with reduced sensitivity to praziquantel, reinforcing the need for alternative schistosomicidal drugs (Fallon and Doenhoff, 1994; Ismail et al., 1994 and 1996, Engels et al., 2002; Caffrey and Secor, 2011). Therefore, parasite biology and host-parasite interaction studies are a feasible strategy to search for new anti-Schistosoma drugs.
As described, S. mansoni presents a complex life cycle that requires two hosts; thus, a better understanding of parasite biology is challenging, demanding, specialized labor, and time consuming (Clegg, 1965; Michaels and Prata, 1968; Basch, 1981; Irie et al., 1987). Inoculation of animal models with parasites is a way to investigate S. mansoni development in the mammalian host, being an approachable procedure and an important system for the evaluation of the complex host-parasite interaction.
Here, we describe a protocol to assess the development of S. mansoni inside a mammalian host. The following procedures detail how to perform a mouse infection with schistosomula and describe methods to evaluate parasite development, such as growth, reproduction, and infection outcome through granuloma analysis in the liver.
Materials and Reagents
1-ml insulin syringe with a 27 G needle (BD, catalog number:309623)
21 G needle (BD, catalog number: 305129)
Plastic Pasteur pipettes (Kasvi, catalog number: K30-300S)
Petri dishes (Sarstedt, catalog number: 82.1473.001)
6-well plates (Sarstedt, catalog number: 83.3920.300)
50-ml conical centrifuge tubes (Sarstedt, catalog number: 62.547.254)
15-ml conical centrifuge tubes (Sarstedt, catalog number: 62.554.205)
Scalpel (CP Lab Safety, catalog number: QC-793-140-EA)
1-200 µl universal tips (Axygen, catalog number: T-200-Y)
100-1,000 µl universal tips (Axygen, catalog number: T-1000-B)
Maxymum Recovery® 0.6-ml microfuge tubes (Axygen, catalog number: MCT-060-L-C)
0.6-ml microfuge tubes (Axygen, catalog number: MCT-060-C-S)
1.5-ml microfuge tubes (Axygen, catalog number: MCT-150-C-S)
Microscope slides, 26 × 76 mm (KASVI, catalog number: K5-7105)
Microscope slide coverslips, 24 × 24 mm (KASVI, catalog number: K5-2424)
Microscope slide coverslips, 24 × 50 mm (KASVI, catalog number: K5-2450)
High-profile disposable blades 818 (Leica Biosystems, catalog number: 14035838383)
Biopsy cassette (CRALPLAST, catalog number: 2921)
Female Swiss Webster mice, 28 days old
Glasgow Minimum Essential Medium (GMEM) (Sigma-Aldrich, catalog number: G6148)
Glucose (Sigma-Aldrich, catalog number: G5767)
Lactalbumin (Sigma-Aldrich, catalog number: L7252)
HEPES (Sigma-Aldrich, catalog number: H3375)
Fetal bovine serum (Thermo Fisher Scientific, Gibco, catalog number: 16000044)
MEM vitamin solution (Thermo Fisher Scientific, Gibco, catalog number: 16000044)
Schneider’s Insect Medium (Sigma-Aldrich, catalog number: S0146)
Triiodothyronine (Sigma-Aldrich, catalog number: 709611)
Hypoxanthine (Sigma-Aldrich, catalog number: H9636)
Hydrocortisone (Sigma-Aldrich, catalog number: H0888)
Penicillin/streptomycin (Thermo Fisher Scientific, Gibco, catalog number: 15070063)
Sodium heparin (Fisher Scientific, Thermo Fisher Scientific, catalog number: BP252410)
NaCl (Merck Millipore, catalog number: 1064040500)
Xylazine hydrochloride (Sigma-Aldrich, catalog number: X1251)
Ketamine hydrochloride (Sigma-Aldrich, catalog number: 343099)
Sodium thiopental (Sigma-Aldrich, catalog number: T6023)
KOH (Sigma-Aldrich, catalog number: 221473)
Absolute ethanol (Merck Millipore, catalog number 64175)
36% formaldehyde (Sigma-Aldrich, catalog number: 47608)
Glacial acetic acid (Merck Millipore, catalog number: 64197)
HCl (Merk Millipore, catalog number: 109057)
Carmine (Sigma-Aldrich, catalog number: C1022)
Methyl salicylate (Sigma-Aldrich, catalog number: M6752)
Canada balsam (Sigma-Aldrich, catalog number: 60610)
N-butyl acetate (Sigma-Aldrich, catalog number: 442666-U)
Histological paraffin (Easypath, catalog number: EP-21-20068A)
Hematoxylin (Sigma-Aldrich, catalog number: H3136)
Eosin (Sigma-Aldrich, catalog number: E4009)
Xylene (Sigma-Aldrich, catalog number: 534056)
Entellan (Merck Millipore, catalog number: 1079610100)
Autoclaved tap water
Autoclaved balanced feed for mice
0.85% NaCl (see Recipes)
10% KOH (see Recipes)
1× PBS (see Recipes)
4% buffered formaldehyde (see Recipes)
10% formaldehyde (see Recipes)
Alcohol-formalin-acetic Acid (AFA) (see Recipes)
70% ethanol (see Recipes)
80% ethanol (see Recipes)
90% ethanol (see Recipes)
95% ethanol (see Recipes)
Hydrochloric carmine (see Recipes)
Hydrochloric alcohol (see Recipes)
1:2 methyl salicylate-Canada balsam (see Recipes)
Supplemented GMEM (see Recipes)
Equipment
Tweezers (CP Lab Safety, catalog number: QC-788-100-EA)
Dissecting scissors (CP Lab Safety, catalog number: QC-791-107-EA)
Perfusion system (AutoMate Scientific, catalog number: 11-140)
200-ml glass beaker
5-mm sieve
Manual 2-20 µl single-channel pipette (Eppendorf, catalog number: 3120000038)
Manual 20-200 µl single-channel pipette (Eppendorf, catalog number: 3120000054)
Manual 100-10,00 µl single-channel pipette (Eppendorf, catalog number: 3120000062)
Eppendorf® Centrifuge 5804R (Sigma-Aldrich, catalog number: EP022628146)
Tissue processing PT05 TS (Lupetec)
Inclusion Center CI 2014 (Lupetec)
Aluminum inclusion molds (Lupetec)
Microtome MRP2015 (Lupetec)
Light microscope Axiostar Plus (Zeiss)
Zeiss microscope Stemi SV 11 (Zeiss)
Inverted microscope Axio Vert (Zeiss)
Inverted microscope Eclipse Ti-E (Nikon) with Confocal C2 plus (Nikon)
Software
ImageJ (Schneider et al., 2012) (https://imagej.net/ImageJ1)
NIS-Elements software (Nikon)
Procedure
Notes:
All mouse procedures must be performed according to the animal facility and national guidelines for animal use.
Prior to and during the infection period, mice were maintained with autoclaved water and autoclaved balanced feed “ad libidum”.
Inoculation of mice with schistosomula
Note: The following procedure is performed using the schistosomula stage (Figure 1). However, mouse inoculation and further procedures can also be performed using the cercariae stage.
Calculate the volume of medium containing 350 schistosomula. To calculate the number of parasites/volume, multiply 350 by the total volume of the culture medium and divide this result by the total number of parasites cultivated in that culture medium.
Note: The number of schistosomula to be inoculated per mouse can be up to 450 parasites per animal. Include a control group.
Pipette the previously calculated volume into a Petri dish or onto microscope slides.
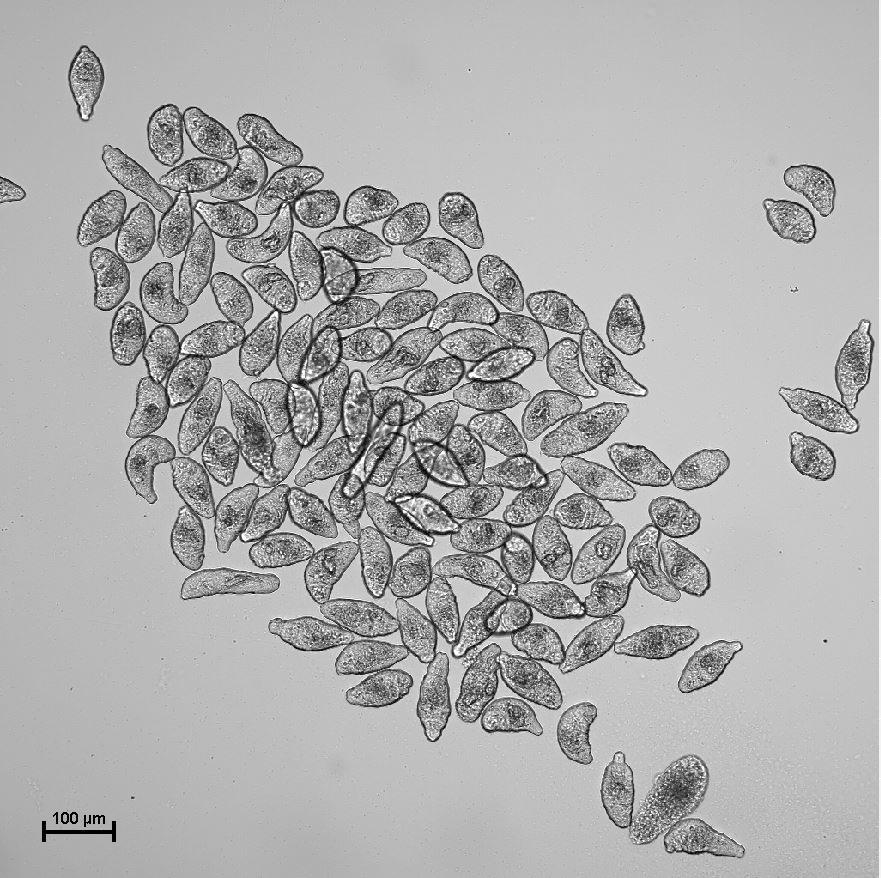
Figure 1. Examples of schistosomula cultivated in culture medium. Representative image of a schistosomula culture obtained at 10× magnification.Withdraw a sample to check, under the microscope, whether the pipetted volume contains the expected number of schistosomula.
Place the parasites from each group into ten Maxymum Recovery® 0.6-ml microfuge tubes (one tube per mouse). Each microfuge tube should contain 350 schistosomula.
Note: In the absence of Maxymum Recovery® microfuge tubes, it is possible to use a sterile microfuge tube instead; however, the use of Maxymum Recovery® microfuge tubes ensures that parasite inoculation will be more homogeneous, since it reduces the number of parasites remaining on the walls of the tube.
Make up the volume to 200-300 µl with supplemented GMEM, if necessary.
Note: The maximum volume to be inoculated into each mouse is 300 µl.
Separate ten female Swiss Webster mice per group.
Note: The mouse strain can be changed according to the experimental question.
Use a 1-ml insulin syringe with a 21 G needle for each mouse.
Fill the syringe with the schistosomula previously added to the 0.6-ml microfuge tube.
Note: Use one syringe and needle per animal to avoid inoculation of a disproportionate number of parasites.
Hold the mouse by grasping the skin along its neck.
Inoculate the parasites through the subcutaneous route by injection of the respective syringe contents into the loose skin over the neck.
Euthanasia and perfusion
Notes:
Since oviposition starts after 35 days of infection, euthanasia should be performed from 40 days after parasite inoculation.
The anesthetics and euthanasia procedures described in this protocol are approved by Brazilian national guidelines following Law 11794/08. The amount and type of sedatives and euthanasia methods should follow the animal use guidelines of each country.
Prepare 1.5 L 0.85% NaCl solution per ten animals.
Add 2,500 U/L sodium heparin to the 0.85% NaCl solution.
Prepare 200 ml 4% buffered formaldehyde solution per ten animals.
Prepare a 2.5% sodium thiopental solution.
Weigh each mouse.
Hold the mouse by grasping the skin along its neck.
Using a 1-ml insulin syringe with a 27 G needle, administer 10 mg/kg xylazine hydrochloride and 150 mg/kg ketamine hydrochloride through the intraperitoneal route by injection into the thigh muscles directed away from the femur.
Wait for the mouse to become anesthetized.
Position the mouse with the abdomen facing upward.
Using a 1-ml insulin syringe with a 27 G needle, administer 150 mg/kg 2.5% sodium thiopental through the intraperitoneal route by injection into the abdomen toward the head.
Press the mouse's feet with tweezers to confirm euthanasia by noting the absence of a movement response.
Clean the abdomen with 70-75% ethanol.
Note: Perfusion was conducted according to Pellegrino and Siqueira (1956) with some modifications.
Using dissecting scissors make an incision along the midline of the abdomen.
Using dissecting scissors, cut the mouse's diaphragm and rib to expose the heart.
Using dissecting scissors, cut the portal vein.
Place the mouse intestine and portal vein in the 200-ml glass beaker.
Insert the needle of the perfusion equipment into the left ventricle.
Start the perfusion equipment for adult worm recovery by NaCl injection.
Note: All the liquid that comes out of the portal vein must fall into the beaker since it carries adult worms.
Stop the perfusion equipment when the liquid from the portal vein becomes clear.
Pass the obtained liquid through a 5-mm sieve, which will retain the recovered adult worms.
Transfer the recovered adult worms from the sieve to a 6-well plate. To perform this procedure, turn the sieve upside down and place it on the well. Using a plastic Pasteur pipette and 0.85% NaCl, wash the sieve to release all the worms into the well.
Cover the adult worms with 0.85% NaCl solution.
Remove the mouse intestine and cut it 3-cm downstream from the end of the ileum.
Place the ileum on a microscope slide, cover with a 24 × 24 mm coverslip, and press.
Place the remaining intestine portion in a a 6-well plate.
Remove the mouse liver.
Remove the median lobe and place into a 50-ml conical centrifuge tube previously filled with 200 ml 4% buffered formaldehyde.
Place the remainder of the liver in a 6-well plate.
Repeat this procedure for all animals.
Note: Label the containers according to the animal: liver, intestine, and worms, and the microscope slide of the ileum.
Assessment of the number of recovered adult worms
Place the recovered adult worms from each mouse in a Petri dish.
Separate the males from the females using a needle or tweezers.
Note: To differentiate males from females, the size and color of the parasites should be observed. Male adult worms are clear and female adult worms are longer and darker. To recognize the male gynecophoric canal and tubercles, which are characteristics absent in the female, it is necessary to use a magnifier light or light microscope.
Count the males and females recovered from each mouse.
Repeat the procedure for the adult worms recovered from the remaining mice.
Analysis of adult worm length
After counting, transfer the female and male adult worms from each mouse to a 1.5-ml microfuge tube filled with 1 ml AFA solution.
Note: Adult worms can be maintained in AFA solution for more than a year.
Randomly transfer one female and one male recovered from the same mouse to a microscope slide.
Stretch the adult worms carefully.
Under a Zeiss Microscope Stemi SV 11, take an image of the adult worms.
Notes:
All images should be taken using the same parameters.
Indicative adult worm images for analysis of length are represented in the original research paper: Tavares, N. C., Gava, S. G., Torres, G. P., de Paiva, C. E. S., Moreira, B. P., Lunkes, F. M. N., Montresor, L. C., Caldeira, R. L. and Mourao, M. M. (2020). Schistosoma mansoni FES Tyrosine Kinase Involvement in the Mammalian Schistosomiasis Outcome and Miracidia Infection Capability in Biomphalaria glabrata.Front Microbiol 11: 963 (37). Link to access:https://www.frontiersin.org/articles/10.3389/fmicb.2020.00963/full.
Transfer the worms back to the 1.5-ml microfuge tube filled with 1 ml AFA solution.
Note: If the microscope does not have a scale, take an image of a ruler under the microscope using the same parameters as those for the worms.
Repeat the procedure for the adult worms recovered from the remaining mice.
Note: The number of adult worms to be analyzed will depend on the total number of adult worms recovered. Perform this procedure using the maximum number of adult worms as possible.
Staining adult worms for confocal analysis
Note: Adult worms are stained and analyzed according to Neves et al. (1998 and 2002).
Use a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette to remove all the AFA solution (Figure 2A).
Fill the 1.5-ml microfuge tube containing the worms with hydrochloric carmine using a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette (Figure 2B).
Optional: Adult worms can be transferred to a small Petri dish or another container that enables parasite soaking in the hydrochloric carmine.
Note: The volume of hydrochloric carmine added should completely cover the worms.
Incubate the worms in hydrochloric carmine for 30 min at room temperature (Figure 2B).
Use a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette to remove the excess hydrochloric carmine (Figure 2B).
Add 70% ethanol and quickly remove the ethanol using a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette (Figure 2C).
Note: This step is important to remove the excess hydrochloric carmine and should be performed quickly to avoid complete removal of hydrochloric carmine.
Repeat Step E5 (Figure 2C).
Add hydrochloric alcohol and, after a few seconds, remove it using a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette (Figure 2C).
Note: This step provides contrast of different organs and must be performed quickly to avoid complete hydrochloric carmine removal. The removal of hydrochloric alcohol should occur when the worms turn from an intense red to a pale red.
Add 70% ethanol and incubate for 5 min at room temperature (Figure 2C).
Use a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette to remove the 70% ethanol (Figure 2C).
Add 80% ethanol and incubate for 5 min at room temperature (Figure 2C).
Use a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette to remove the 80% ethanol (Figure 2C).
Add 95% ethanol and incubate for 5 min at room temperature (Figure 2C).
Use a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette to remove the 95% ethanol (Figure 2C).
Add absolute ethanol and incubate for 5 min at room temperature (Figure 2C).
Use a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette to remove the absolute ethanol (Figure 2C).
Notes:
When pipetting, care should be taken to avoid worm disruption.
The added volume of hydrochloric alcohol and the subsequent ethanol washes should always cover the worms.
Steps E5-E14 can be performed using a small Petri dish or another container. The stained worms can be soaked in the solutions using a 5-mm sieve.
Use a 20-200 µl manual single-channel or a plastic Pasteur pipette to add 1:2 methyl salicylate-Canada balsam solution for at least 24 h. Cover all the worms with the solution and incubate for at least 24 h (Figure 2D and Figure 3A).
Note: Adult worms can be maintained in this solution for years.
Place the male and female worms separately onto microscope slides (Figure 2E).
Cover the adult worms with 1:2 methyl salicylate-Canada balsam solution using a 20 µl-200 µl manual single-channel pipette or a plastic Pasteur pipette (Figure 2E).
Carefully cover the microscope slide with a 24 × 24 mm coverslip (Figure 2E).
Fill the space between the microscope slide and the coverslip with 1:2 methyl salicylate-Canada balsam solution using a 20-200 µl manual single-channel pipette or a plastic Pasteur pipette (Figure 2E).
Place the slides on a flat surface to dry (Figure 3B).
Note: The microscope slides should rest on a flat surface to dry for 1-2 months. Observe every day whether more 1:2 methyl salicylate-Canada balsam solution should be added to avoid drying out.
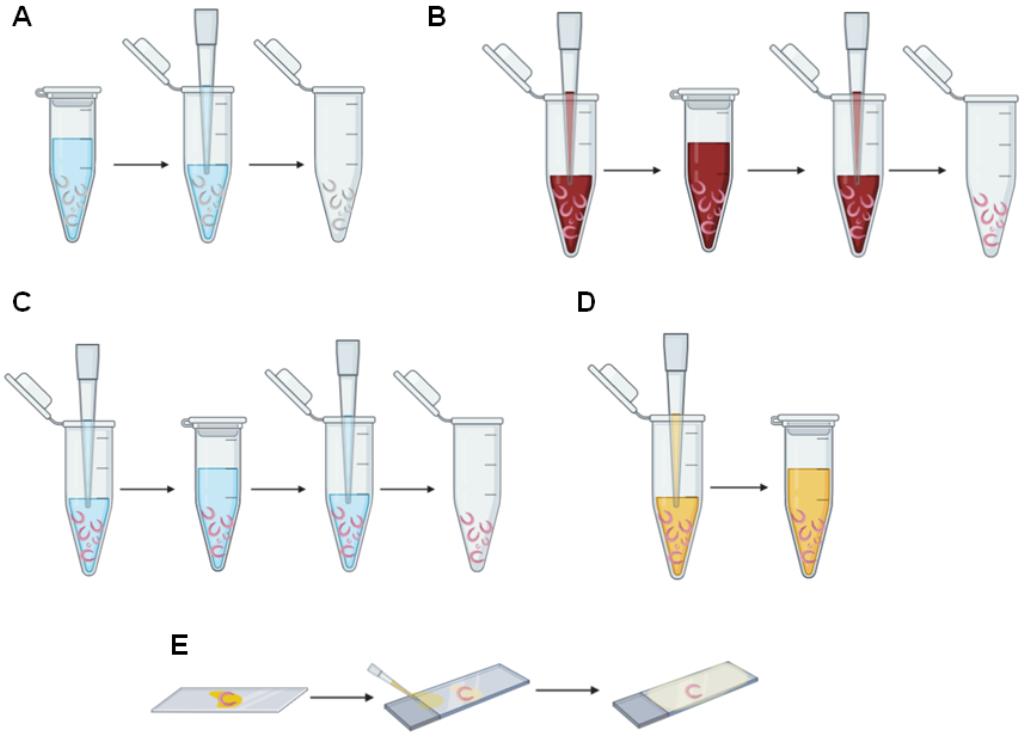
Figure 2. Schema of staining adult worms for confocal analysis. A. Removal of AFA solution using a manual single-channel pipette. B. Hydrochloric carmine addition, incubation, and removal using a manual single-channel pipette. C. Addition, incubation, and removal of hydrochloric alcohol, 70%, 80%, 95%, and absolute ethanol using a manual single-channel pipette. D. Addition of 1:2 methyl salicylate-Canada balsam solution using a manual single-channel pipette. E. Assembly of slides of stained adult worms.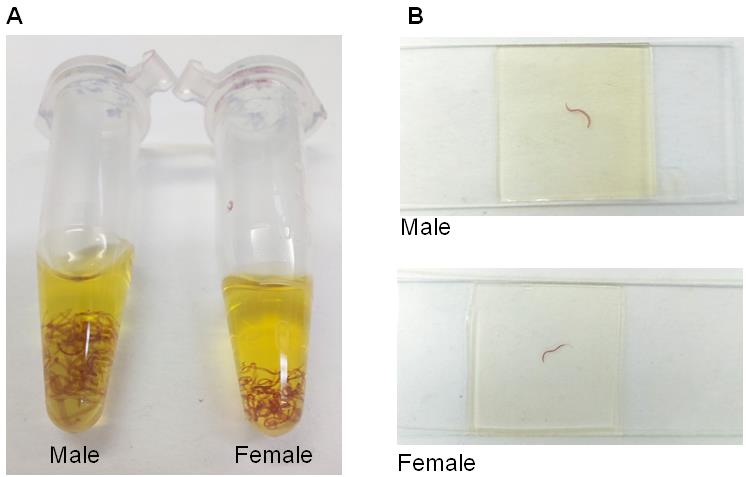
Figure 3. Example of stained adult worms. A. Male and female adult worms stained with hydrochloric carmine and covered with 1:2 methyl salicylate-Canada balsam solution. B Microscope slides containing male and female adult worms stained with hydrochloric carmine.
Liver and intestine digestion and egg recovery
Place the remaining liver and intestine of one animal into two different Petri dishes and slice the organs using a scalpel (Figure 4A).
Transfer the sliced liver to a 15-ml Falcon tube (Figure 4A).
Perform this procedure for all mice livers and intestines, separately.
Add 10 ml 10% KOH to dissolve the liver and intestinal tissue; this will not damage the eggs (Figure 4B).
Incubate the livers and intestines in 10% KOH at 4 °C overnight (Figure 4B).
Vortex the tubes (Figure 4C).
Incubate the tubes at 37 °C for at least 1 hour (Figure 4C).
Note: If the livers and intestines are not completely dissolved, vortex again and incubate the tubes until the organs are completely dissolved.
Centrifuge the tubes for 5 min at 380 × g (Figure 4D).
Discard the supernatant (Figure 4D).
Add 10 ml 0.85% NaCl, resuspend the pellet using a plastic Pasteur pipette, and centrifuge the tubes for 5 min at 380 × g (Figure 4D).
Perform Steps F8-F10 three times (Figure 4D).
After the final centrifugation, discard the supernatant and resuspend the pellet in 1 ml 0.85% NaCl (Figure 4E).
Transfer the contents to a clean 1.5-ml microfuge tube (Figure 4E).
For counting, homogenize the tubes and pipette 10 µl contents to a microscope slide (Figure 4F).
Under a light microscope, count the eggs observed at 5× magnification and record the number (Figure 4F).
Perform Steps F14 and F15 three times.
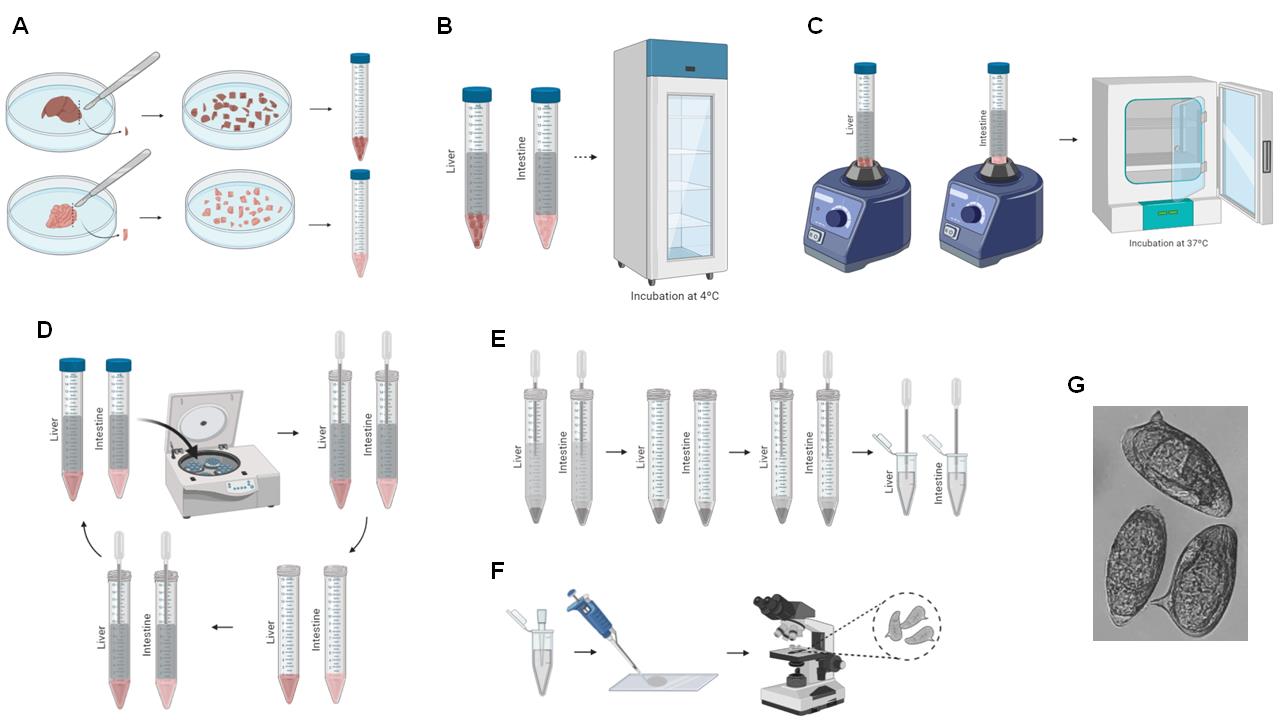
Figure 4. Schema of liver and intestine digestion for egg recovery. A. Liver and intestine are sliced using a scalpel, and the chopped organs are transferred to a 15-ml Falcon tube (Steps F1-F2). B. 10% KOH is added to the tissue and incubated overnight (Steps F3-F5). C. The tissue is vortexed and incubated at 37 °C (Steps F6-F7). D. The 15-ml Falcon tube is centrifuged, the supernatant is removed, and 1 ml 0.85% NaCl is added (Steps F8-F11). E. The resuspended eggs are transferred to a 1.5-ml microfuge tube (Steps F12-F13). F. The recovered eggs are counted (Steps F14-F16). G. Representative image of recovered eggs obtained at 10× magnification.
Oogram
Place the microscope slides of the ileum on a light microscope.
For the first 100 eggs observed, count the mature, immature, and dead eggs.
Consider as immature, the eggs from the first to the fourth maturation stage as described by Mati and Melo (2013).
Note: You can also classify the eggs by stage (1-5, with 5 being an egg containing the developed miracidium; known as mature).
Histology for granuloma analysis
Place the median lobe, previously fixed in 4% buffered formaldehyde, in a Petri dish.
Slice the median lobe into six fragments using a scalpel.
Transfer the fragments to a biopsy cassette.
Keep the remaining portion of liver in 4% buffered formaldehyde.
Close the biopsy cassette.
Label the biopsy cassettes according to the respective animals.
Place the biopsy cassettes into the tissue processing PT05 TS (Lupetec).
Fill the tissue processing PT05 TS (Lupetec) buckets with the respective solution/reagents: 10% formaldehyde, 70% ethanol, 95% ethanol, absolute ethanol, N-butyl acetate, xylene, and histological paraffin.
Set the following steps on the tissue processing PT05 TS (Lupetec).
10% formaldehyde for 1 h.
10% formaldehyde for 1 h.
70% ethanol for 1 h.
95% ethanol for 1 h.
95% ethanol for 1 h.
Absolute ethanol for 1 h.
Xylene I for 1 h.
Xylene II for 1 h.
Xylene III for 1 h.
Paraffin I for 1 h.
Paraffin II for 1 h.
Note: Histological processing can be performed manually following the steps described in Step H9.
Dispense a few drops of histological paraffin onto an aluminum inclusion mold.
Transfer the liver slices from the biopsy cassettes to the mold.
Using the Inclusion Center CI 2014 (Lupetec), dispense histological paraffin onto the liver fragment.
Fit the respective biopsy cassette (the bottom part of the cassette, which contains the identification) on the mold.
Transfer to the chilled plate of the Inclusion Center CI 2014 (Lupetec).
Perform Steps H1-H14 for the other liver samples.
Remove the blocks from the aluminum inclusion molds.
Incubate the blocks at room temperature overnight.
Note: Paraffin blocks containing tissue can be maintained at room temperature until microscope slide assembly.
Incubate the blocks at -20 °C for 1 h.
Place the blocks on ice.
Fit each block on the Microtome MRP2015 (Lupetec)。
Section the blocks to obtain thin slices (approximately 4-6 µM).
Place the slices in a 50 °C water bath.
Note: This step is important to stretch the slices; the temperature cannot be higher since the paraffin melts.
Capture the slices with a microscope slide.
Label the microscope slides.
Incubate the microscope slides at 65 °C for 4 h.
Fill four containers with xylene and label each container with: xylene I, xylene II, xylene III, and xylene IV.
Fill four containers with absolute ethanol and label each with: absolute ethanol I, absolute ethanol II, absolute ethanol III, and absolute ethanol IV.
Fill a container with hematoxylin.
Fill a container with eosin.
Dip the slides in xylene I for 10 min.
Dip the slides in xylene II for 10 min.
Dip the slides in absolute ethanol I for 10 s.
Dip the slides in absolute ethanol II for 10 s.
Wash the slides with running water for 3 min.
Dip the slides in hematoxylin for 5 min.
Dip the slides in eosin for 30 s.
Wash the slides with water for 2 s.
Wash the slides with absolute ethanol III for 10 s.
Wash the slides with absolute ethanol IV for 10 s.
Incubate the slides at 37 °C for at least 15 min.
Dip the slides in xylene III for 10 s.
Dip the slides in xylene IV for 10 s.
Using a plastic Pasteur pipette, add two drops of entellan to the middle of the slide, making two separate circles.
Dip the extremity of the slide in xylene.
Fit the 24 × 50 mm microscope slide coverslips.
Slightly press the coverslip against the slide.
Incubate the slide at room temperature until completely dry.
Note: Slides can be stored for years at room temperature.
Place the dry slide on the inverted microscope Axio Vert (Zeiss).
Adjust the 10× objective.
Locate the granuloma.
Take an image of the granuloma.
Note: For a better reproduction, take images from exudative-productive granulomas.
Take images of 100 granulomas per group.
Notes:
This number can be modified according to the number of granulomas found.
All images should be taken using the same parameters.
Data analysis
Length analysis of adult worms
Open the image (with a known distance according to the parameters used to take images of adult worms in ImageJ.
Select the “line selection tool” and draw a line to a known distance.
Select “Analyze” and then “Set scale”.
Type the known distance and its respective unit, select “Global”, and then select “ok”.
Open the worm image in ImageJ.
Select “Analyze” and then “Set Measurements”.
Select “length”.
Select the “line selection tool” and draw a line to the worm.
Select “Analyze” and then “Measure”.
Note: If the worms are not straight, carefully draw a line to each part of the worm and then sum the values to obtain the entire worm length.
Perform the analysis for all images.
Number of eggs recovered from the liver and intestine
Calculate the mean number of eggs from triplicate samples; perform the assessment as described above (Procedure F).
The mean value multiplied by 100 gives the number of eggs per ml (the total number of eggs per animal, recovered from the liver or intestine, will depend on the volume).
Add together the number of eggs recovered from the liver and intestine to yield the total number of eggs per animal.
Fecundity of female adult worms
To obtain fecundity data, divide the total number of eggs by the number of females recovered from the respective animal.
Oogram analysis
Calculate the percentage of mature, immature, and dead eggs found in each mouse ileum.
Analysis of the granuloma area
Open ImageJ.
Open an image with a known distance.
Set the scale as described in steps 2-4 of Data analysis B.
Open the granuloma image in ImageJ.
Select “Analyze” and then “Set Measurements”.
Select “area”.
Select the “freehand selection tool” and carefully circle the granuloma.
Select “Analyze” and then “Measure”.
Perform this analysis for all granulomas.
Note: Indicative results of data analysis A, B, C, D, and E, and the procedures C and E are represented in the original research paper Tavares, N. C., Gava, S. G., Torres, G. P., de Paiva, C. E. S., Moreira, B. P., Lunkes, F. M. N., Montresor, L. C., Caldeira, R. L. and Mourão, M. M. (2020).Schistosoma mansoni FES Tyrosine Kinase Involvement in the Mammalian Schistosomiasis Outcome and Miracidia Infection Capability in Biomphalaria glabrata.Front Microbiol 11: 963 (37). Link to access: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00963/full.
Recipes
Note: All the following recipes can be stored at room temperature.
0.85% NaCl
8.5 g NaCl
Make up to 1 L with distilled water
Note: Prepare at least 1.5 L per 10 mice.
10% KOH
100g KOH
Make up to 1 L with distilled water
1× PBS
137 mM NaCl
2.7 mM KCl
10 mM Na2HPO4
1.8 mM KH2PO4
pH 7.4
4% buffered formaldehyde
111.11 ml formaldehyde
888.89 ml 1× PBS
10% formaldehyde
277.77 ml formaldehyde
722.23 ml distilled water
Alcohol-formalin-acetic Acid (AFA)
95 ml absolute ethanol
3 ml formaldehyde
2 ml acetic acid
70% ethanol
700 ml absolute ethanol
300 ml distilled water
80% ethanol
800 ml absolute ethanol
200 ml distilled water
90% ethanol
900 ml absolute ethanol
100 ml distilled water
95% ethanol
950 ml absolute ethanol
50 ml distilled water
Hydrochloric carmine
5 g carmine
5 ml HCl
5 ml distilled water
Make up to 200 ml with 90% ethanol
Note: Crush the carmine and add to the HCl and water. Place in a water bath for 1 h. Allow to cool. Make up the volume to 200 ml with 90% ethanol.
Hydrochloric alcohol
100 ml ethanol
0.5 ml HCl
1:2 methyl salicylate-Canada balsam
50 ml methyl salicylate
100 ml Canada balsam
Supplemented GMEM
50 ml Glasgow Minimum Essential Medium (GMEM) (prepared and filtered according to the manufacturer’s instructions)
0.1% glucose
0.1% lactalbumin
20 mM HEPES
2% inactivated fetal bovine serum
0.5% MEM vitamin solution
5% Schneider’s Insect Medium
0.5 μM hypoxanthine
1 μM hydrocortisone
1% penicillin/streptomycin
Note: Store at 4 °C.
Acknowledgments
The authors are grateful for funding from the European Commission’s Seventh Framework Programme for Research, under Grant Agreement no. 602080 (A-ParaDDisE), FAPEMIG (CBB-APQ-0520-13), CAPES Programa PCDD-Programa CAPES/Nottingham University (003/2014), the Productivity Fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) granted to MMM (302518/2018-5), and the fellowship to NCT from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001. The authors would also like to thank the Snail and Animal Facility of the René Rachou Institute-Fiocruz for supplying parasites and mice, and the Program for Technological Development in Tools for Health-PDTIS/FIOCRUZ for the use of its facilities.
Competing interests
The authors have no conflicts of interest.
Ethics
The presented animal study was approved by Brazilian national guidelines following Law 11794/08 and by the Ethics Commission for Animal Use (CEUA) of the Oswaldo Cruz Foundation under the number LM-05/18.
References
- Armstrong, J. C. (1965). Mating Behavior and Development of Schistosomes in the Mouse. J Parasitol 51: 605-616.
- Basch, P. F. (1981). Cultivation of Schistosoma mansoni in vitro. I. Establishment of cultures from cercariae and development until pairing. J Parasitol 67(2): 179-185.
- Burke, M. L., Jones, M. K., Gobert, G. N., Li, Y. S., Ellis, M. K. and McManus, D. P. (2009). Immunopathogenesis of human schistosomiasis. Parasite Immunol 31(4): 163-176.
- Caffrey, C. R. and Secor, W. E. (2011). Schistosomiasis: from drug deployment to drug development. Curr Opin Infect Dis 24(5): 410-417.
- Clegg, J. A. (1965). In Vitro Cultivation of Schistosoma mansoni. Exp Parasitol 16: 133-147.
- de Waard. and Vermeulen, N. E. (1961). Penetration of the mammalian skin by cercariae of Schistosoma mansoni. Trop Geogr Med 13: 82-88.
- Engels, D., Chitsulo, L., Montresor, A. and Savioli, L. (2002). The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop 82(2): 139-146.
- Espírito Santo, M. C., Azeredo, L. M., Teles, H. M., Gryschek, R. C., Ferreira, C. S. and Amato Neto, V. (2008). Abdominal ultrasound in the evaluation of fibrosis and portal hypertension in an area of schistosomiasis low endemicity. Rev Inst Med Trop Sao Paulo 50(2): 117-119.
- Fallon, P. G. and Doenhoff, M. J. (1994). Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg 51(1): 83-88.
- Gryseels, B., Polman, K., Clerinx, J. and Kestens, L. (2006). Human schistosomiasis. Lancet 368(9541): 1106-1118.
- Hitchcock, D. J. (1949). Penetration characteristics of Schistosoma mansoni cercariae. J Parasitol 35(2): 216.
- Irie, Y., Tanaka, M. and Yasuraoka, K. (1987). Degenerative changes in the reproductive organs of female schistosomes during maintenance in vitro. J Parasitol 73(4): 829-835.
- Ismail, M. M., Taha, S. A., Farghaly, A. M. and el-Azony, A. S. (1994). Laboratory induced resistance to praziquantel in experimental schistosomiasis. J Egypt Soc Parasitol 24(3): 685-695.
- Ismail, M., Metwally, A., Farghaly, A., Bruce, J., Tao, L. F. and Bennett, J. L. (1996). Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am J Trop Med Hyg 55(2): 214-218.
- King, C. H. and Dangerfield-Cha, M. (2008). The unacknowledged impact of chronic schistosomiasis. Chronic Illn 4(1): 65-79.
- Mati, V. L. and Melo, A. L. (2013). Current applications of oogram methodology in experimental schistosomiasis; fecundity of female Schistosoma mansoni and egg release in the intestine of AKR/J mice following immunomodulatory treatment with pentoxifylline. J Helminthol 87(1): 115-124.
- Michaels, R. M. and Prata, A. (1968). Evolution and characteristics of Schistosoma mansoni eggs laid in vitro. J Parasitol 54(5): 921-930.
- Miller, P. and Wilson, R. A. (1980). Migration of the schistosomula of Schistosoma mansoni from the lungs to the hepatic portal system. Parasitology 80(2): 267-288.
- Moore, D. V. and Sandground, J. H. (1956). The relative egg producing capacity of Schistosoma mansoni and Schistosoma japonicum. Am J Trop Med Hyg 5(5): 831-840.
- Neves, R. H., Oliveira, S. A., Machado-Silva, J. R., Coutinho, E. and Gomes, D. C. (2002). Phenotypic characterization of Schistosoma mansoni adult worms recovered from undernourished mice: a morphometric study focusing on the reproductive system. Rev Soc Bras Med Trop 35(4): 405-407.
- Neves, R. H., Pereira, M. J., de Oliveira, R. M., Gomes, D. C. and Machado-Silva, J. R. (1998). Schistosoma mansoni Sambon, 1907: morphometric differences between adult worms from sympatric rodent and human isolates. Mem Inst Oswaldo Cruz 93 Suppl 1: 309-312.
- Olveda, D. U., Inobaya, M., Olveda, R. M., Vinluan, M. L., Ng, S. K., Weerakoon, K., McManus, D. P., Ramm, G. A., Harn, D. A., Li, Y., Lam, A. K., Guevarra, J. R. and Ross, A. G. (2017). Diagnosing schistosomiasis-induced liver morbidity: implications for global control. Int J Infect Dis 54: 138-144.
- Pellegrino, J. and Siqueira, A. F. (1956). [A perfusion technic for recovery of Schistosoma mansoni from experimentally infected guinea pigs]. Rev Bras Malariol Doencas Trop 8(4): 589-597.
- Schneider, C. A., Rasband, W. S. and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7): 671-675.
- Stirewalt, M. A. (1974). Schistosoma mansoni: cercaria to schistosomule. Adv Parasitol 12: 115-182.
- Tavares, N. C., Gava, S. G., Torres, G. P., de Paiva, C. E. S., Moreira, B. P., Lunkes, F. M. N., Montresor, L. C., Caldeira, R. L. and Mourao, M. M. (2020). Schistosoma mansoni FES Tyrosine Kinase Involvement in the Mammalian Schistosomiasis Outcome and Miracidia Infection Capability in Biomphalaria glabrata. Front Microbiol 11: 963.
- Thétiot-Laurent, S. A., Boissier, J., Robert, A. and Meunier, B. (2013). Schistosomiasis chemotherapy. Angew Chem Int Ed Engl 52(31): 7936-7956.
- Uberos, J., and Jerez-Calero, A. (2012). “Imported Schistosomiasis,” in Schistosomiasis. Pediatrics Service, Universitary Hospital "San Cecilio", Granada, Spain. ISBN: 978-953-307-852-6.
- Watson, M. (2009). Praziquantel. J Exot Pet Med 3(18): 229-231.
- World Health Organization. (2018). Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2016. 2019-05-03.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Tavares, N. C. and Mourão, M. M. (2021). Parasitemia Evaluation in Mice Infected with Schistosoma mansoni. Bio-protocol 11(10): e4017. DOI: 10.21769/BioProtoc.4017.
Category
Microbiology > Microbe-host interactions
Immunology > Animal model > Mouse
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









