- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Unbiased Screening of Activated Receptor Tyrosine Kinases (RTKs) in Tumor Extracts Using a Mouse Phospho-RTK Array Kit
Published: Vol 9, Iss 8, Apr 20, 2019 DOI: 10.21769/BioProtoc.3216 Views: 5678
Reviewed by: Chiara AmbrogioValerian DORMOYAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Detection of ALT Associated Promyelocytic Leukemia Nuclear Bodies (APBs) by Immunofluorescence-FISH (IF-FISH)
Siamak A. Kamranvar and Maria G. Masucci
Dec 5, 2014 14444 Views

Dual Phospho-CyTOF Workflows for Comparative JAK/STAT Signaling Analysis in Human Cryopreserved PBMCs and Whole Blood
Ilyssa E. Ramos [...] James M. Cherry
Nov 20, 2025 2336 Views
Abstract
Kaposi’s sarcoma (KS) herpesvirus (KSHV) is a virus that causes KS, an angiogenic AIDS-associated spindle-cell neoplasm, by activating host oncogenic signaling cascades through autocrine and paracrine mechanisms. Many host signaling cascades co-opted by KSHV including PI3K/AKT/mTORC, NFkB and Notch are critical for cell-specific mechanisms of transformation and their identification is paving the way to therapeutic target discovery. Analysis of the molecular KS signature common to human KS tumors and our mouse KS-like tumors showed consistent expression of KS markers VEGF and PDGF receptors with upregulation of other angiogenesis ligands and their receptors in vivo. This points to the autocrine and paracrine activation of various receptor tyrosine kinase (RTK) signaling axes. Hereby we describe a protocol to screen for activated receptor tyrosine kinase of KSHV-induced KS-like mouse tumors using a Mouse Phospho-RTK Array Kit and its validation by RTK western blots. We showed that this method can be successfully used to rank the tyrosine kinase receptors most activated in tumors in an unbiased manner. This allowed us to identify PDGFRA as an oncogenic driver and therapeutic target in AIDS-KS.
Keywords: PDGFRABackground
Kaposi’s sarcoma herpesvirus (KSHV) is the etiological agent of Kaposi’s sarcoma (KS) (Chang et al., 1994; Ganem, 2010; Mesri et al., 2010; Dittmer and Damania, 2016). KS is a major cancer associated with AIDS (AIDS-KS) and is the most prevalent type of cancer affecting men and children in Africa (Ganem, 2010; Mesri et al., 2010; Cavallin et al., 2014). Although the incidence of AIDS-KS in the western world has markedly declined since the wide-spread implementation of HAART (Highly Active Antiretroviral Therapy), a significant percentage of AIDS-KS patients never achieve total remission (Krown, 2003; Nguyen et al., 2008; Cavallin et al., 2014). Moreover, KSHV prevalence and KS incidence appear to be increasing, even in HAART treated HIV patients with controlled viremias (Maurer et al., 2007; Labo et al., 2015). Understanding the interplay of viral and host factors in KS oncogenesis is critical for the rational development of new therapies (Dittmer and Krown, 2007; Sullivan et al., 2009). Many host signaling cascades co-opted by KSHV including PI3K/AKT/mTORC, NFkB and Notch are critical for cell-specific mechanisms of transformation and their identification is paving the way to therapeutic target discovery (Sodhi et al., 2006; Emuss et al., 2009; Liu et al., 2010; Mesri et al., 2014; Dittmer and Damania, 2016). Analysis of the molecular KS signature common to human KS tumors and our mouse KS-like tumors, showed consistent expression of KS markers VEGFR 1, 2, 3, Podoplanin with upregulation of angiogenesis ligands and receptors in vivo, pointing to the upregulation of various receptor tyrosine kinase signaling axes (Mutlu et al., 2007; Mesri et al., 2010). Thus, we set out to rank the host tyrosine kinase signaling cascades activated by KSHV using a mouse model of KSHV-dependent tumorigenesis that helped us to identify PDGFRA as an oncogenic driver and therapeutic target in AIDS-KS (Cavallin et al., 2018).
Here we describe an assay protocol using a Mouse Phospho-RTK Array Kit in mouse KSHV-induced KS-like tumors, which can be used as a reliable method to rank the tyrosine kinase receptors most activated in tumors in an unbiased manner. The Proteome Profiler Mouse Phospho-RTK Array Kit (R&D Systems) is a membrane-based sandwich immunoassay. Captured antibodies spotted in duplicate on nitrocellulose membranes bind to specific target proteins present in the sample. Tyrosine phosphorylation of the captured proteins is detected with an HRP-conjugated pan phospho-tyrosine antibody and then visualized using chemiluminescent detection reagents. The signal produced is proportional to the amount phosphorylation in the bound analyte.
Surprisingly, analysis of the pattern of tumor RTK activation (Figures 1 and 2) showed a single prominently activated RTK spot that accounted for a significant portion of the total tyrosine kinase activation of the tumors. This signal corresponded to the PDGF receptor alpha-chain (PDGFRA) (Cavallin et al., 2018). Validation of the array by Western Blot analysis (Figure 3A) shows that PDGFRA is robustly expressed and much more phosphorylated in the tumors than in mouse normal skin. On the other hand, c-kit (Figure 3B), a related RTK, is similarly expressed in skin and tumors and displays low levels of activation in the tumors; that are similar to those in the skin control and correlates with the low activation of c-kit in our phospho-tyrosine kinase proteomic array (Cavallin et al., 2018).
Materials and Reagents
Common lab consumables
- Pipette tips
- Gloves
- Plastic containers with the capacity to hold 50 ml (for washing the arrays)
- Plastic wrap
- Absorbent lab wipes (KimWipes® or equivalent)
- Paper towels
- Plastic transparent sheet protector (trimmed to 10 cm x 12 cm and open on three sides)
- TissueRuptor Disposable Probes (QIAGEN, catalog number: 990890)
Reagent Preparation (Mouse Phospho-RTK Array Kit)
- Proteome Profiler Mouse Phospho-RTK Array Kit (R&D Systems, catalog number: ARY014)
Kit Contents:- 4 Array Membranes
- 4-Well Multi-dish
- Array Buffers:1)Lysis Buffer 17 (see Recipes)2)Array Buffer 13)1x Array Buffer 2 (see Recipes)4)1x Wash Buffer (see Recipes)5)Chemi Reagent Mix (see Recipes)
- Lysis Buffer
- Wash Buffer
- Anti-Phospho-Tyrosine-HRP Detection Antibody
- Chemiluminescent Detection Reagents
- Transparency Overlay Template
- Detailed Protocol
- Aprotinin (Sigma, catalog number: A6279)
- Leupeptin (Tocris, catalog number: 1167)
- Pepstatin (Tocris, catalog number: 1190)
Materials and reagents for Western blots
- PDVF membranes
- CL-XPosureTM Film, 5 x 7 in. (13 x 18 cm) (Thermo Fisher Scientific, catalog number: 34090)
- 4x Laemmli Sample Buffer (Bio-Rad Laboratories, catalog number: 1610747)
- 4%-20% Mini-PROTEAN® TGX Stain-FreeTM Protein Gels, 12-well, 20 µl (Bio-Rad Laboratories, catalog number: 4568095)
- 10x Tris/Glycine Buffer for Western Blots and Native Gels (Bio-Rad Laboratories, catalog number: 1610771)
- 10x Tris/Glycine/SDS (Bio-Rad Laboratories, catalog number: 1610772)
- Methanol ACS reagent, ≥ 99.8% (Sigma, catalog number: 179337)
- PierceTM BCA Protein Assay Kit (Thermo Scientific, catalog number: 23227)
- Blotting-Grade Blocker, nonfat dry milk (Bio-Rad Laboratories, catalog number: 1706404)
- Tween 20, 100% Nonionic Detergent (Bio-Rad Laboratories, catalog number: 1706531)
- TBS Buffer, 20x liquid (VWR, catalog number: 97064-338)
- RestoreTM PLUS Western Blot Stripping Buffer (Thermo Scientific, catalog number: 46430)
- SuperSignalTM West Pico PLUS Chemiluminescent Substrate (Thermo Scientific, catalog number: 34577)
- Primary Antibodies:
cKIT and p-cKIT (Cell Signaling Technology, catalog number: 9370)
PDGFRA and p-PDGFRA (R&D Systems, catalog numbers: AF307 and AF2114) - HRP-labeled secondary antibodies (Promega, catalog number: W4011)
- Deionized or distilled water
- 1x Tris/Glycine running buffer (see Recipes)
- Transfer buffer (see Recipes)
- 1x TBS (1 L) (see Recipes)
- TBS/Tween (see Recipes)
- 5% Blotting-Grade Blocker, nonfat dry milk/TBS/Tween (see Recipes)
Equipment
- Pipettes (Gilson, Pipetman Classic)
- Flat-tipped tweezers
- Autoradiography cassette
- Rocking platform shaker
- TissueRuptor II (QIAGEN, catalog number: 9002755)
- Centrifuge 5810R (Eppendorf, catalog number: 5811000010)
- Film developer Alphatek AX-390-SE (Alphatek)
- Rocking platform shaker
Software
- Protein Array Analyzer for ImageJ (Author: Gilles Carpentier, Faculte des Sciences et Technologies, Universite Paris Est Creteil Val de Marne, France, https://imagej.nih.gov/ij/macros/toolsets/Protein%20Array%20Analyzer.txt)
- GraphPad Prism 7.0a (GraphPad Software, Inc., www.graphpad.com)
Procedure
- Reagent Preparation (Mouse Phospho-RTK Array Kit)
Note: Bring all reagents to room temperature before use.- Mouse Phospho-RTK Array
Four nitrocellulose membranes each containing 39 different anti-RTK antibodies printed in duplicate. Handle arrays only with gloved hands and flat-tipped tweezers. - Anti-Phospho-Tyrosine-HRP Detection Antibody: 50 μl of mouse anti-phospho-tyrosine antibody conjugated to HRP
Immediately before use, dilute the Detection Antibody to the working concentration specified on the vial label using 1x Array Buffer 2.
Note: Prepare only as much Detection Antibody as needed to run each experiment. - Lysis Buffer 17 (see Recipe 5)
- 1x Array Buffer 2 (see Recipe 6)
- 1x Wash Buffer (see Recipe 7)
- Chemi Reagent Mix (see Recipe 8)
- Mouse Phospho-RTK Array
- Sample Preparation
- Excise by dissection and disrupt 1-5 mg of tumor tissue (approx 0.2-0.3 cm3) using TissueRuptor II in 1 ml Lysis Buffer 17 prepared with protease inhibitors.
- Centrifuge samples at 10,621 x g for 10 min at 4 °C in an Eppendorf Centrifuge 5810R.
- Collect supernatant and quantified protein concentrations in tumor lysates using the PierceTM BCA Protein Assay Kit.
- The suggested starting range for tissue lysates is 100-300 μg and the maximum allowable lysate volume is 250 μl/array.
- Tissue lysates should be used immediately or aliquoted and stored at ≤ -70 °C.
- Thawed lysates should be kept on ice prior to use.
- Array Procedure
Note: Bring all reagents to room temperature before use. Keep samples on ice. To avoid contamination, wear gloves while performing the procedures.- Prepare all reagents and samples as directed in the previous sections.
- Pipette 2.0 ml of Array Buffer 1 into each well of the 4-well Multi-dish that will be used. Array Buffer 1 is used as a block buffer.
- Using flat-tip tweezers, remove each array to be used from between the protective sheets.
- Place one array into each well of the 4-well Multi-dish. The array number should be facing upward.
Note: Upon contact with Array Buffer 1 the blue dye will disappear from the spots. The capture antibodies are retained in their specific locations. - Incubate for 1 h at room temperature on a rocking platform shaker. Orient the tray so that each array rocks from end to end in its well.
- While the arrays are blocking, prepare samples by diluting the desired quantity of lysate in 1.25 ml of Array Buffer 1. Adjust to a final volume of 1.5 ml with Lysis Buffer 17 as necessary. The maximum allowable lysate volume is 250 μl/array.
- Aspirate Array Buffer 1 from the 4-well Multi-dish. Add the prepared samples and place the lid on the 4-well Multi-dish.
- Incubate overnight at 2-8 °C on a rocking platform shaker.
Note: A shorter incubation time may be used if optimal sensitivity is not required. - Carefully remove each array and place into individual plastic containers with 20 ml of 1x Wash Buffer. Rinse the 4-well Multi-dish with deionized or distilled water and dry thoroughly.
- Wash each array with 1x Wash Buffer for 10 min on a rocking platform shaker. Repeat two times for a total of three washes.
- Dilute the Anti-Phospho-Tyrosine-HRP Detection Antibody in 1x Array Buffer 2 using the dilution factor on the vial label. Pipette 2.0 ml into each well of the 4-well Multi-dish.
- Carefully remove each array from its wash container. Allow excess buffer to drain from the array. Return the array to the 4-well Multi-dish containing the Anti-Phospho-Tyrosine-HRP and cover with the lid.
- Incubate for 2 h at room temperature on a rocking platform shaker.
- Wash each array as described in Steps C9 and C10.
Note: Complete the remaining steps without interruption. - Carefully remove each membrane from its wash container. Allow excess Wash Buffer to drain from the membrane by blotting the lower edge onto paper towels. Place each membrane on the bottom sheet of the plastic sheet protector with the identification number facing up.
- Pipette 1 ml of the prepared Chemi Reagent Mix evenly onto each membrane.
Note: Using less than 1 ml of Chemi Reagent Mix per membrane may result in incomplete membrane coverage. - Carefully cover with the top sheet of the plastic sheet protector. Gently smooth out any air bubbles and ensure Chemi Reagent Mix is spread evenly to all corners of each membrane. Incubate for 1 min.
- Position paper towels on the top and sides of the plastic sheet protector containing the membranes and carefully squeeze out excess Chemi Reagent Mix.
- Remove the top plastic sheet protector and carefully lay an absorbent lab wipe on top of the membranes to blot off any remaining Chemi Reagent Mix.
- Leaving membranes on the bottom plastic sheet protector, cover the membranes with plastic wrap taking care to gently smooth out any air bubbles. Wrap the excess plastic wrap around the back of the sheet protector so that the membranes and sheet protector are completely wrapped.
- Place the membranes with the identification numbers facing up in an autoradiography film cassette.
Note: Use an autoradiography cassette that is not used with radioactive isotope detection. - Expose membranes CL-XPosureTM Film. Multiple exposure times are recommended (Figure 1).
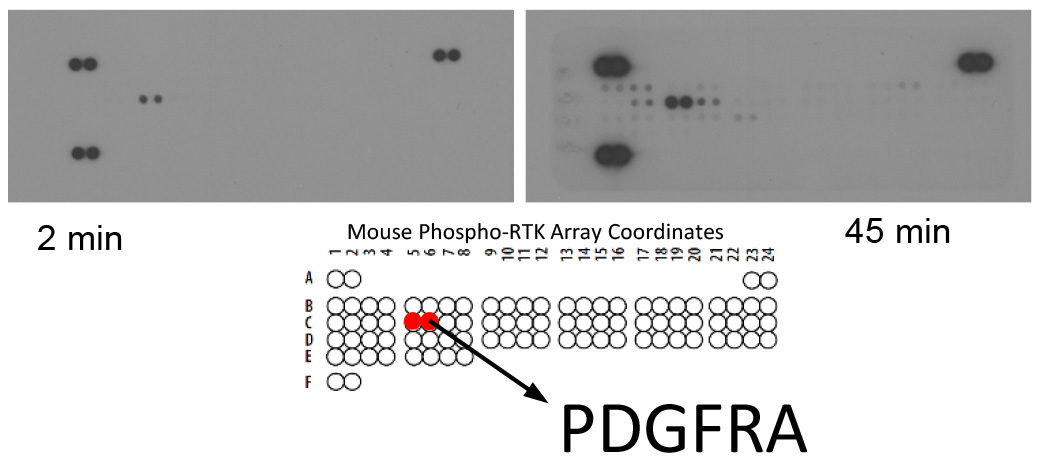
Figure 1. Proteomic analysis of Receptor tyrosine kinases in mouse KSHV-induced KS-like tumors shows activation of PDGF receptor-alpha. Mouse Phospho-Receptor Tyrosine Kinase (RTK) Array Kit used to quantify levels of phosphorylation of 39 RTKs in mouse KSHV-induced KS-like tumors. Note the major activation spot corresponding to PDGF receptor alpha chain (Cavallin et al., 2018).
Data analysis
The positive signals seen on developed film can be quickly identified by placing the transparency overlay template on the array image and aligning it with the pairs of reference spots in three corners of each array. The stamped identification number on the array should be placed on the left-hand side. The location of controls and capture antibodies is listed in the Appendix.
Notes:
- Reference spots are included to align the transparency overlay template and to demonstrate that the array has been incubated with Anti-Phospho-Tyrosine-HRP during the assay procedure.
- Pixel densities on developed X-ray film can be collected and analyzed using a transmission mode scanner and image analysis software.
- Using ImageJ Protein Array Analyzer tool create a template to analyze pixel density in each spot of the array.
- Export signal values to a spreadsheet file for manipulation in a program such as Microsoft Excel or GraphPad Prism.
- Determine the average signal (pixel density) of the pair of duplicate spots representing each RTK.
- Subtract an averaged background signal from each RTK. Use a signal from a clear area of the array or the PBS negative control spots as a background value (Figure 2).
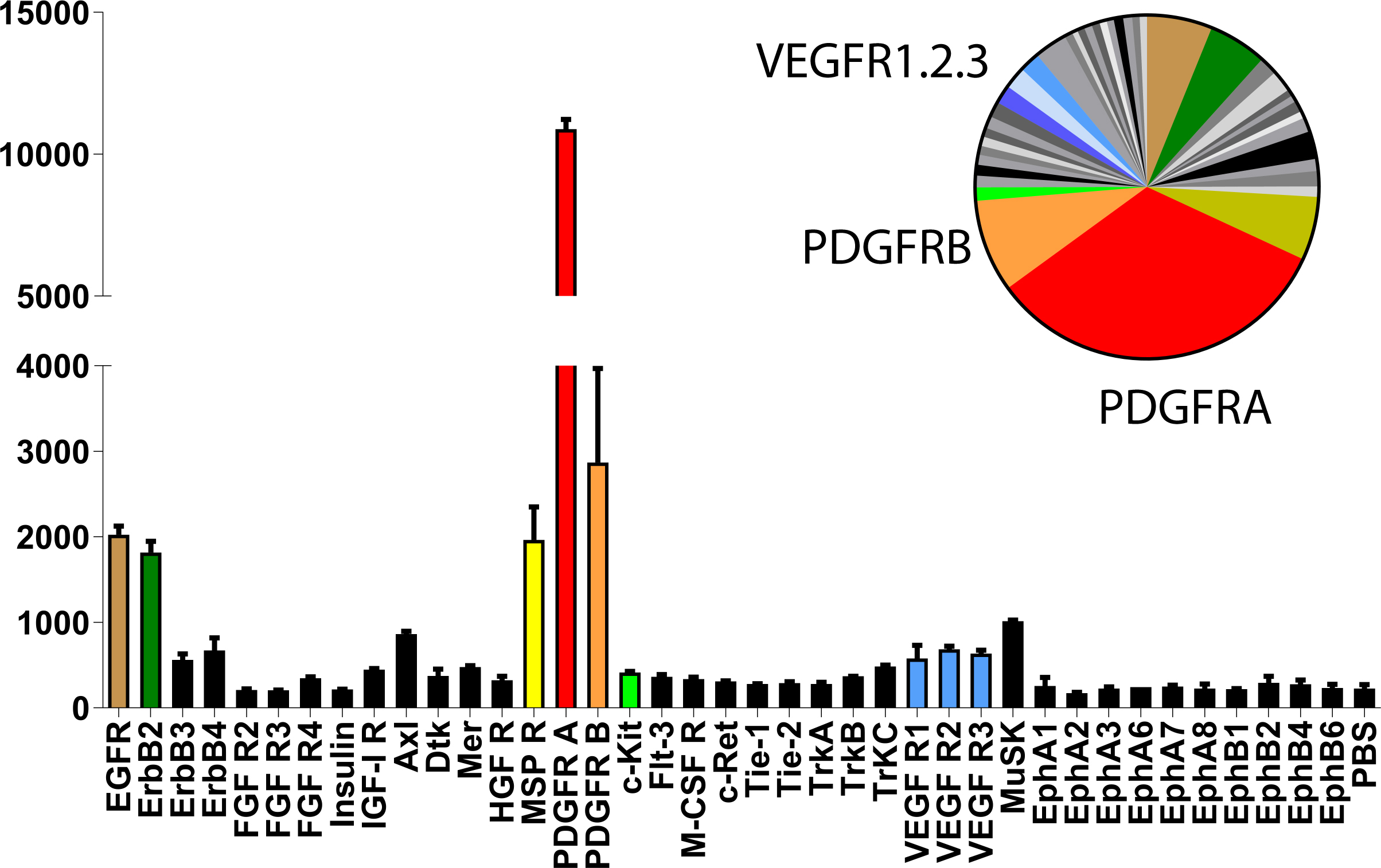
Figure 2. Quantification of the 45 min exposure array from Figure 1. Bar graph and pie chart from densitometry for the higher-exposure blot are equally color coded for the most prominent signals (Cavallin et al., 2018).
Western Blotting
Twenty micrograms of proteins of the same tumor lysates used for the Mouse Phospho-RTK Array were mixed with Laemmli buffer, boiled for 5 min, resolved by SDS-PAGE in 4-20% Mini-PROTEAN® TGX Stain-FreeTM Protein Gels, 12-well. Proteins were transferred to polyvinylidene fluoride (PVDF) membrane by blotting overnight at 300 mA. Membranes were blocked with 5% nonfat milk/TBS/Tween 20 for 1 h and incubated with primary antibodies (4 °C, 16 h). After 3 TBS/Tween 20 washes, membranes were incubated with HRP-labeled secondary antibodies (1:10,000 dilution) for 1 h at room temperature. Protein bands were developed using SuperSignalTM West Pico PLUS Chemiluminescent Substrate. To analyze multiple proteins on the same membrane, membranes were washed with Restore PLUS Western Blot Stripping Buffer according to the manufacturer’s protocol (Figure 3).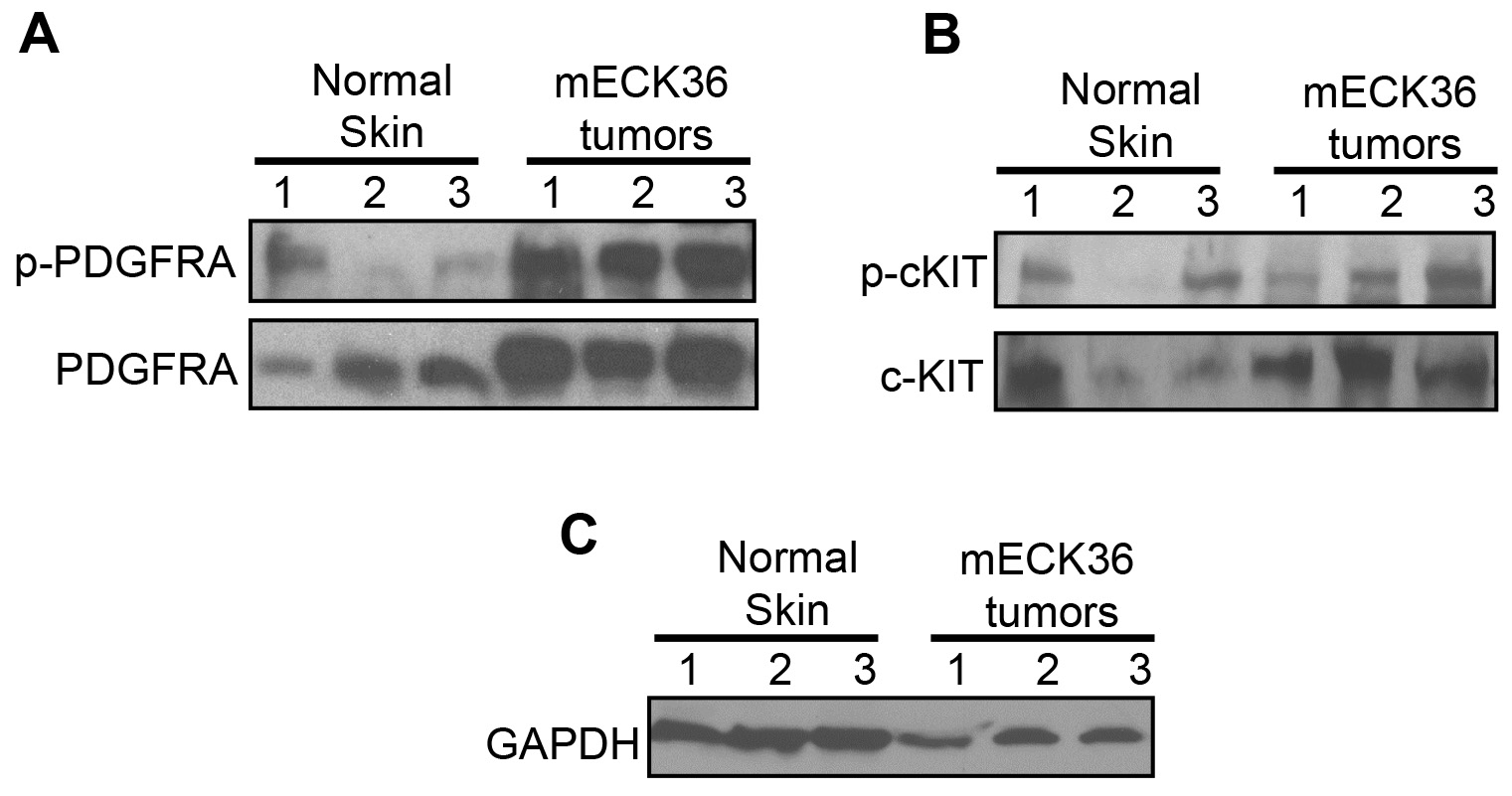
Figure 3. Validation of the mouse Phospho-receptor Tyrosine Kinase (RTK) array kit. PDGFRA and phospho-PDGFRA (A) or c-KIT and phospho-cKIT (B) determined in 3 different samples of Mouse Normal Skin and mouse KSHV-induced KS-like tumors from 3 different mice by immunoblotting (Cavallin et al., 2018). (C) GAPDH used as loading control.
Recipes
- 1x Tris/Glycine running buffer (1 L)
100 ml 10x Tris/Glycine
900 ml distilled water - Transfer buffer (1 L)
100 ml of 10x Tris/Glycine/SDS
200 ml Methanol
700 ml distilled water - 1x TBS (1 L)
50 ml 20x TBS
950 ml distilled water - TBS/Tween (500 ml)
25 ml TBS 20x
1 ml Tween 20
Distilled water to 500 ml - 5% Blotting-Grade Blocker, nonfat dry milk/TBS/Tween (10 ml)
500 mg Blotting-Grade Blocker, nonfat dry milk
10 ml TBS/Tween
Note: Bring all reagents to room temperature before use.
- Lysis Buffer 17
Add 10 μg/ml Aprotinin, 10 μg/ml Leupeptin, and 10 μg/ml Pepstatin to the volume of Lysis Buffer 17 required for cell lysate preparation
Prepare fresh for each use - 1x Array Buffer 2
Add 2 ml of Array Buffer 2 Concentrate to 8 ml of deionized or distilled water
Prepare fresh for each use - 1x Wash Buffer
If crystals have formed in the concentrate, warm the bottles to room temperature and mix gently until the crystals have completely dissolved
Dilute 40 ml of 25x Wash Buffer Concentrate into 960 ml of deionized or distilled water - Chemi Reagent Mix
Chemi Reagents 1 and 2 should be mixed in equal volumes within 15 min of use (protect from light)
1 ml of the resultant mixture is required per membrane
Acknowledgments
This work was supported by NIH grants CA75918, and CA136387; and by NCI/OHAM supplements from the Miami CFAR grant 5P30AI07396, and by the Florida Biomedical Foundation, Bankhead Coley Foundation Grant 3BB05.
Competing interests
The authors do not have any conflicts of interests or competing interests.
Ethics
The animal experiments have been performed under UM IACUC approval number 13±093. The University of Miami has an Animal Welfare Assurance on file with the Office of Laboratory Animal Welfare (OLAW), National Institutes of Health. Additionally, UM is registered with USDA APHIS. The Council on Accreditation of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC International) has continued the University of Miami's full accreditation.
References
- Cavallin, L. E., Goldschmidt-Clermont, P. and Mesri, E. A. (2014). Molecular and cellular mechanisms of KSHV oncogenesis of Kaposi's sarcoma associated with HIV/AIDS. PLoS Pathog 10(7): e1004154.
- Cavallin, L. E., Ma, Q., Naipauer, J., Gupta, S., Kurian, M., Locatelli, P., Romanelli, P., Nadji, M., Goldschmidt-Clermont, P. J. and Mesri, E. A. (2018). KSHV-induced ligand mediated activation of PDGF receptor-alpha drives Kaposi's sarcomagenesis. PLoS Pathog 14(7): e1007175.
- Chang, Y., Cesarman, E., Pessin, M. S., Lee, F., Culpepper, J., Knowles, D. M. and Moore, P. S. (1994). Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266(5192): 1865-1869.
- Dittmer, D. P. and Damania, B. (2016). Kaposi sarcoma-associated herpesvirus: immunobiology, oncogenesis, and therapy. J Clin Invest 126(9): 3165-3175.
- Dittmer, D. P. and Krown, S. E. (2007). Targeted therapy for Kaposi's sarcoma and Kaposi's sarcoma-associated herpesvirus. Curr Opin Oncol 19(5): 452-457.
- Emuss, V., Lagos, D., Pizzey, A., Gratrix, F., Henderson, S. R. and Boshoff, C. (2009). KSHV manipulates Notch signaling by DLL4 and JAG1 to alter cell cycle genes in lymphatic endothelia. PLoS Pathog 5(10): e1000616.
- Ganem, D. (2010). KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J Clin Invest 120(4): 939-949.
- Krown, S. E. (2003). Therapy of AIDS-associated Kaposi's sarcoma: targeting pathogenetic mechanisms. Hematol Oncol Clin North Am 17(3): 763-783.
- Labo, N., Miley, W., Benson, C. A., Campbell, T. B. and Whitby, D. (2015). Epidemiology of Kaposi's sarcoma-associated herpesvirus in HIV-1-infected US persons in the era of combination antiretroviral therapy. AIDS 29(10): 1217-1225.
- Liu, R., Li, X., Tulpule, A., Zhou, Y., Scehnet, J. S., Zhang, S., Lee, J. S., Chaudhary, P. M., Jung, J. and Gill, P. S. (2010). KSHV-induced notch components render endothelial and mural cell characteristics and cell survival. Blood 115(4): 887-895.
- Maurer, T., Ponte, M. and Leslie, K. (2007). HIV-associated Kaposi's sarcoma with a high CD4 count and a low viral load. N Engl J Med 357(13): 1352-1353.
- Mesri, E. A., Cesarman, E. and Boshoff, C. (2010). Kaposi's sarcoma and its associated herpesvirus. Nat Rev Cancer 10(10): 707-719.
- Mesri, E. A., Feitelson, M. A. and Munger, K. (2014). Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe 15(3): 266-282.
- Mutlu, A. D., Cavallin, L. E., Vincent, L., Chiozzini, C., Eroles, P., Duran, E. M., Asgari, Z., Hooper, A. T., La Perle, K. M., Hilsher, C., Gao, S. J., Dittmer, D. P., Rafii, S. and Mesri, E. A. (2007). in vivo-restricted and reversible malignancy induced by human herpesvirus-8 KSHV: a cell and animal model of virally induced Kaposi's sarcoma. Cancer Cell 11(3): 245-258.
- Nguyen, H. Q., Magaret, A. S., Kitahata, M. M., Van Rompaey, S. E., Wald, A. and Casper, C. (2008). Persistent Kaposi sarcoma in the era of highly active antiretroviral therapy: characterizing the predictors of clinical response. AIDS 22(8): 937-945.
- Sodhi, A., Chaisuparat, R., Hu, J., Ramsdell, A. K., Manning, B. D., Sausville, E. A., Sawai, E. T., Molinolo, A., Gutkind, J. S. and Montaner, S. (2006). The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell 10(2): 133-143.
- Sullivan, R. J., Pantanowitz, L. and Dezube, B. J. (2009). Targeted therapy for Kaposi sarcoma. BioDrugs 23(2): 69-75.
Article Information
Copyright
© 2019 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Naipauer, J., Cavallin, L. E. and Mesri, E. A. (2019). Unbiased Screening of Activated Receptor Tyrosine Kinases (RTKs) in Tumor Extracts Using a Mouse Phospho-RTK Array Kit. Bio-protocol 9(8): e3216. DOI: 10.21769/BioProtoc.3216.
Category
Cancer Biology > Proliferative signaling > Biochemical assays > Protein analysis
Microbiology > in vivo model > Viruses
Molecular Biology > Protein > Phosphorylation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link