- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Fabrication and Use of the Dual-Flow-RootChip for the Imaging of Arabidopsis Roots in Asymmetric Microenvironments
Published: Vol 8, Iss 18, Sep 20, 2018 DOI: 10.21769/BioProtoc.3010 Views: 9654
Reviewed by: Tie LiuJonathan GilkersonArun Shunmugam

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Novel Imaging Protocol for Investigating Arabidopsis thaliana Siliques and Seeds Using X-rays
Brylie A. Ritchie [...] Ansul Lokdarshi
Oct 5, 2023 2182 Views

Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Pradeep Chand Deo
Oct 20, 2025 1425 Views

CAPS-Based SNP Genotyping for Nitrogen-Response Phenotypes in Maize Hybrids
Jannis Jacobs [...] Peter K. Lundquist
Dec 20, 2025 543 Views
Abstract
This protocol provides a detailed description of how to fabricate and use the dual-flow-RootChip (dfRootChip), a novel microfluidic platform for investigating root nutrition, root-microbe interactions and signaling and development in controlled asymmetric conditions. The dfRootChip was developed primarily to investigate how plants roots interact with their environment by simulating environmental heterogeneity. The goal of this protocol is to provide a detailed resource for researchers in the biological sciences wishing to employ the dfRootChip in particular, or microfluidic devices in general, in their laboratory.
Keywords: Dual-flow-RootChipBackground
Conditions belowground are highly heterogeneous and dynamic, hence plant roots are exposed to various stimuli and consequently have to adapt to this complex environment. Despite the importance of these developmental adaptations, the underlying mechanisms still remain to be elucidated. Microfluidic devices have proven useful to cultivate specimens in controlled microenvironments and facilitate access for live imaging of dynamic processes from the subcellular to the organismic level (Crane et al., 2010). Thanks to the possibilities of microfluidics to manipulate small fluid volumes in a controlled manner, conduct experiments in high-throughput, extract quantitative information and perform time-lapse measurements, microfluidic devices have found their way into organismal studies. For the model plant Arabidopsis thaliana, a series of microfluidic devices have been developed that enable the monitoring of gene expression during root development (Busch et al., 2012), signaling events (Keinath et al., 2015) and sensor-based imaging of nutrient uptake (Grossmann et al., 2011; Lanquar et al., 2014). Additionally, more recent advances using microfluidic platforms have included high-resolution phenotyping (Jiang et al., 2014; Xing et al., 2017) and the investigation of root-microbe interactions (Parashar and Pandey, 2011; Massalha et al., 2017). While the root microenvironment can be precisely controlled in these perfusion devices, environmental complexity, a hallmark of natural root environments, was challenging to simulate (Stanley et al., 2016; Stanley and van der Heijden, 2017). The dfRootChip was therefore developed to enable the study of single Arabidopsis roots in asymmetric microenvironments at the cellular level to investigate gene expression, signaling and development (Stanley et al., 2018). Importantly, the dfRootChip can be implemented in a range of applications, which include performing localized treatments with drugs, differential nutrient or stress conditions, probing host-microbe interactions (e.g., pathogenic and beneficial interactions, potential biocontrol agents), and investigating root physiology and root hair development.
The current protocol was developed to provide fundamental know-how to researchers wishing to implement this platform. This protocol therefore provides a detailed explanation of how to fabricate the dfRootChip, using photo- and soft lithography, and how to cultivate Arabidopsis seedlings within the dfRootChip. Due to the wide applicability of microfluidics in biology, a number of steps in this protocol will also aid the fabrication and handling of other device designs. Furthermore, this protocol illustrates how the dfRootChip can be utilized in three different experimental settings. Specifically, we highlight how to perform (i) symmetric and asymmetric root treatments over longer time-periods (hours to days), (ii) localized inoculation of plant roots with bacteria and (iii) rapid asymmetric treatments with the dfRootChip. We exemplify these applications by utilizing different phosphate treatments, the bacterium Pseudomonas fluorescens and a calcium elicitor treatment respectively.
Materials and Reagents
Note: Catalog numbers are provided for commercial, non-custom-made products (see Note 1).
- Polyester film photolithography mask (Micro Lithography Services Ltd. UK, custom-made)
- 100 mm silicon wafers (Silicon Materials)
- SU8 3050 photoresist (MicroChem)
- Plastic cups (Semadeni, catalog number: 8323 )
- Plastic spatulas (Semadeni, catalog number: 3340 )
- Glass coverslips, 75 mm x 50 mm, No. 1 (Th. Geyer, catalog number: 11678524 )
- Cutting blades (Häberle Labortechnik, catalog number: 9156110 )
- Scotch® MagicTM Invisible tape (3M)
- Microcentrifuge tubes 1.5 ml (Eppendorf Safe-Lock, Eppendorf, catalog number: 0030120086 )
- Sterilised filter tips 100-1,000 μl (Pipetman Diamond Tips D1000ST, Gilson, catalog number: F171501 )
- 0.2 μm sterile syringe filters (Lab Logistic Group, catalog number: 9.055 511 )
- Sterilised filter tips 0.1-20 μl (Pipetman Diamond Tips DL10ST, Gilson, catalog number: F171201 )
- Sterilised filter tips 2-200 μl (Pipetman Diamond Tips D200ST, Gilson, catalog number: F171301 )
- 94 mm diameter sterile Petri dishes (HUBERLAB, catalog number: 7.663 161 )
- Parafilm® (Bemis, HUBERLAB, catalog number: 15.1550.01 )
- Silicone tubing (TYGON® 0.020” ID x 0.062” OD; type ND-100-80) (Th. Geyer, catalog number: AAD04103 )
- Gauge 23 dosage needles with Luer lock fitting (Gonano Dosiertechnik, catalog number: IP423050-EAR-BULK )
- Syringes 20 ml (VWR, BD PlastipakTM, catalog number: 613-3922 )
- Rotilabo-screw neck ND24 vials (Carl Roth, catalog number: LC88.1 )
- Screw caps with bore hole (Carl Roth, catalog number: LC97.1 )
- Septa Ø 22 mm, ND24, 1.6 mm, 55° (Carl Roth, catalog number: LC98.1 )
- Mini 3-way stopcock, 2 x Luer female, 1 x Luer male (NeoLab, catalog number: 270124190 )
- Male-male luer connectors (Vygon, catalog number: 893.00 )
- Polystyrene cuvettes (SARSTEDT, catalog number: 67.742 )
- 120 x 120 mm2 Petri dishes, sterile (Carl Roth, catalog number: PX67.1 )
- Aluminum foil (can be purchased from any supermarket)
- Arabidopsis thaliana seeds; lines are chosen based on individual needs
- Optional: Pseudomonas fluorescens WCS365-GFP strain (Haney et al., 2015)
- mrDev-600 developer solution (Micro Resist Technology)
- Isopropyl alcohol [(CH3)2CHOH] (Sigma-Aldrich, catalog number: W292907 )
- Chlorotrimethylsilane [(CH3)3SiCl] (Sigma-Aldrich, catalog number: 92361 )
- Sylgard 184 Kit [Poly(dimethylsiloxane), PDMS] (Biesterfeld Helvetia, catalog number: 5498840000 )
- Acetone (CH3COCH3) (Sigma-Aldrich, catalog number: 00560 )
- Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 71687 )
- Ethanol (EtOH) (Sigma-Aldrich, catalog number: 51976 )
- Deionised water, purified by reverse osmosis or ultrafiltration; referred to as "purified water" below
- Sodium hypochlorite 14% Cl2 in aqueous solution (NaClO) (VWR, catalog number: 90350.5000 )
- Micro agar (Duchefa Biochemie, catalog number: M1002.1000 )
- Hoagland’s No. 2 Basal Salt Mixture (Sigma-Aldrich, catalog number: H2395-10L )
- MES hydrate (C6H13NO4S•xH2O) (Sigma-Aldrich, catalog number: M8250 )
- Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: P5958 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- Potassium dihydrogen phosphate (KH2PO4) (AppliChem, catalog number: A3620 )
- Magnesium sulfate heptahydrate (MgSO4•7H2O) (Merck, catalog number: 1.05886.1000 )
- Potassium nitrate (KNO3) (Sigma-Aldrich, catalog number: 31263 )
- Ethylenediaminetetraacetic acid disodium salt dihydrate (Na2EDTA•2H2O) (AppliChem, catalog number: A3553 )
- Calcium nitrate tetrahydrate (Ca(NO3)2•4H2O) (Sigma-Aldrich, Fluka, catalog number: 21197 )
- Boric acid (H3BO3) (Sigma-Aldrich, catalog number: B6768 )
- Copper(II) sulfate pentahydrate (CuSO4•5H2O) (Grüssing, catalog number: 12079 )
- Zinc sulfate (ZnSO4) (Sigma-Aldrich, catalog number: Z1001 )
- Sodium molybdate (Na2MoO4) (Sigma-Aldrich, catalog number: 243655 )
- Manganese chloride dihydrate (MnCl2•2H2O) (Grüssing, catalog number: 12097 )
- Cobalt(II) chloride hexahydrate (CoCl2•6H2O) (Carl Roth, catalog number: T889 )
- Potassium chloride (KCl) (AppliChem, catalog number: A3582 )
- Luria Bertani broth (Sigma-Aldrich, catalog number: L3022 )
- Kanamycin sulfate (Carl Roth, catalog number: T832.3 )
- ½x Hoagland’s medium (½x HM) (see Recipes)
- ½x Hoagland’s agar medium (see Recipes)
- Phosphate rich medium (see Recipes)
- Phosphate deficient medium (see Recipes)
- Lysogeny broth (LB) medium (see Recipes)
- 100 mM salt solution (see Recipes)
Equipment
- Duran® laboratory bottles 500 ml (DWK Life Sciences, DURAN, catalog number: 21 801 44 5 )
- Duran® laboratory bottles 1,000 ml (DWK Life Sciences, DURAN, catalog number: 21 801 54 5 )
- Metal pins (New England Small Tube, catalog number: NE-1310-02 )
- Schott® culture tubes, 160 mm x 16 mm (DWK Life Sciences, DURAN, catalog number: 26 135 21 5 )
- Compressed air (Oil-free compressor, 9 L, 8 bar) (e.g., IMPLOTEX, catalog number: NEW-325 )
- Glass beakers (HUBERLAB, catalog number: 9.0112.43 )
- Forceps (VWR, RGS Solingen, catalog number: 232-0078 )
- Precision air regulator (Ashcroft, Ingersoll-Rand, catalog number: PR4021200 )
- 250 ml Erlenmeyer flask (Sigma-Aldrich, DWK Life Sciences, DURAN, catalog number: Z232815 )
- Spatula (HUBERLAB, catalog number: 13.1556.05 )
- Vortex (HUBERLAB, catalog number: 17.1378.01 )
- Pipettes (Pipetman classic P1000, Gilson, catalog number: F123602 )
- Pipettes (Pipetman classic P200, Gilson, catalog number: F123601 )
- Pipettes (Pipetman classic P10, Gilson, catalog number: F144802 )
- pH meter (Mettler-Toledo International, model: FiveEasyTM FE20 )
- Autoclave (Systec, model: VX-75 )
- Drying oven (SalvisLab, model: VC 20 )
- Standard refrigerator (4-6 °C)
- Biosafety cabinet equipped with UV (Kojair, model: Kojair® SilverLine BlueSeries 200 )
- Precision balance (Mettler-Toledo International, model: PB3001 )
- Analytical balance (Mettler-Toledo International, model: AB104-S/FACT )
- Spin coater (SAWATEC, model: SM-180-BM )
- Hot plate (SAWATEC, model: HP 160 III BM )
- 125 mm x 125 mm x 2 mm soda lime glass plate (Willi Möller)
- Mask aligner (Karl Süss, model: MA6 )
- Custom-made plastic chip holder (frame with an aperture for the RootChip and outer dimensions that fit the microscope stage)
- Incubator (Memmert, model: UM400 )
- Incubator with flask shaker (Eppendorf, New BrunswickTM, model: Innova® 44 )
- Wet bench with filtered laminar air flow (Goller Reinraum)
- Spin coater (Laurell Technologies, model: WS-650-23 )
- Utility knife
- Hole puncher (Syneo, model: Accu-Punch MP10 )
- Hole punch (cutting edge diameter, 0.71 mm) (Syneo, catalog number: CR0350255N20R4 )
- Hole punch (cutting edge diameter, 1.02 mm) (Syneo, catalog number: CR0500355N18R4 )
- Hole punch (cutting edge diameter, 4.75 mm) (Syneo, catalog number: HS1871730P1183S )
- Ultrasonic cleaner (BANDELIN electronic, catalog number: 301 )
- Plasma cleaner (Diener electronic, model: FEMTO40kHZ )
- Vacuum pump (Pfeiffer Vacuum, catalog number: PK D56 712 )
- Vacuum desiccator (Thermo Fisher Scientific, catalog number: 5311-0250 )
- Centrifuge (Thermo Fisher Scientific, model: HeraeusTM PicoTM 21 , catalog number: 75002553)
- Climate chamber (Panasonic, PHC, model: MLR-352H )
- Stratalinker® UV crosslinker (Stratagene, model: 1800 , catalog number: 400071)
- Syringe pumps (World Precision Instruments, model: AL-6000 )
- Stereo microscope (Nikon, model: SMZ1270 )
- Spectrophotometer (GE Healthcare, NovaspecTM Plus, catalog number: 80-2117-50 )
- Inverted microscope (any model, specific to user and application)
- Optional: source of pressurized, clean, dry air
Software
- AutoCAD Mechanical 2011 (AutoDesk, USA)
- Fiji (Schindelin et al., 2012)
Procedure
- Master mould fabrication (process outlined in Figure 1; see Note 1)
- Coat a 100 mm diameter silicon wafer with SU-8 3050 photoresist using a spin coater (SAWATEC). The film thickness of approximately 115 μm can be achieved by adapting the spin speed according to the photoresist manufacturer specifications (see Note 2).
- Place the photoresist-coated wafer onto a hot plate at 95 °C and bake for 20 min (soft bake, see Note 2). A larger silicon wafer or a sheet of aluminum foil can be used to protect the hotplate from contamination by the photoresist.
- Attach the photolithography film mask to a 125 mm soda-lime glass plate and, using a mask aligner with a built-in ultra-violet light source, pattern the dfRootChip design onto the photoresist by exposing the resist to an ultra-violet light source with an exposure energy of 500 mJ/cm2 (λ = 365 nm) (see Notes 2 and 3). The function of the soda-lime glass plate is to carry the mask and to hold it rigid to ensure good contact with the resist.
- Bake the photoresist on a hot plate at 95 °C for 20 min (post-exposure bake, see Note 2).
- To develop the structures (i.e., remove the unexposed photoresist), completely immerse the silicon wafer in an SU-8 developer for ca. 15 min with agitation (mr-Dev 600 developer solution). Then, rinse the structures with fresh developer solution for ca. 10 sec (see Note 2).
- Rinse the master mould with isopropyl alcohol for ca. 10 sec and air-dry thoroughly. To ensure that the structures are completely dry, use filtered, compressed air (see Note 2). If a white film is produced when rinsing, the master mould has been under developed. If this is the case, immerse the master mould in fresh developer solution until the film has been removed and rinse again with IPA.
- Place the master mould and an open glass vial containing 50 μl of chlorotrimethylsilane inside a vacuum desiccation chamber. Next, evacuate the chamber by applying a vacuum pressure of 50 mbar, then close the valve to seal the chamber and allow the master mould to incubate in the chlorotrimethylsilane vapor for at least 1 h.
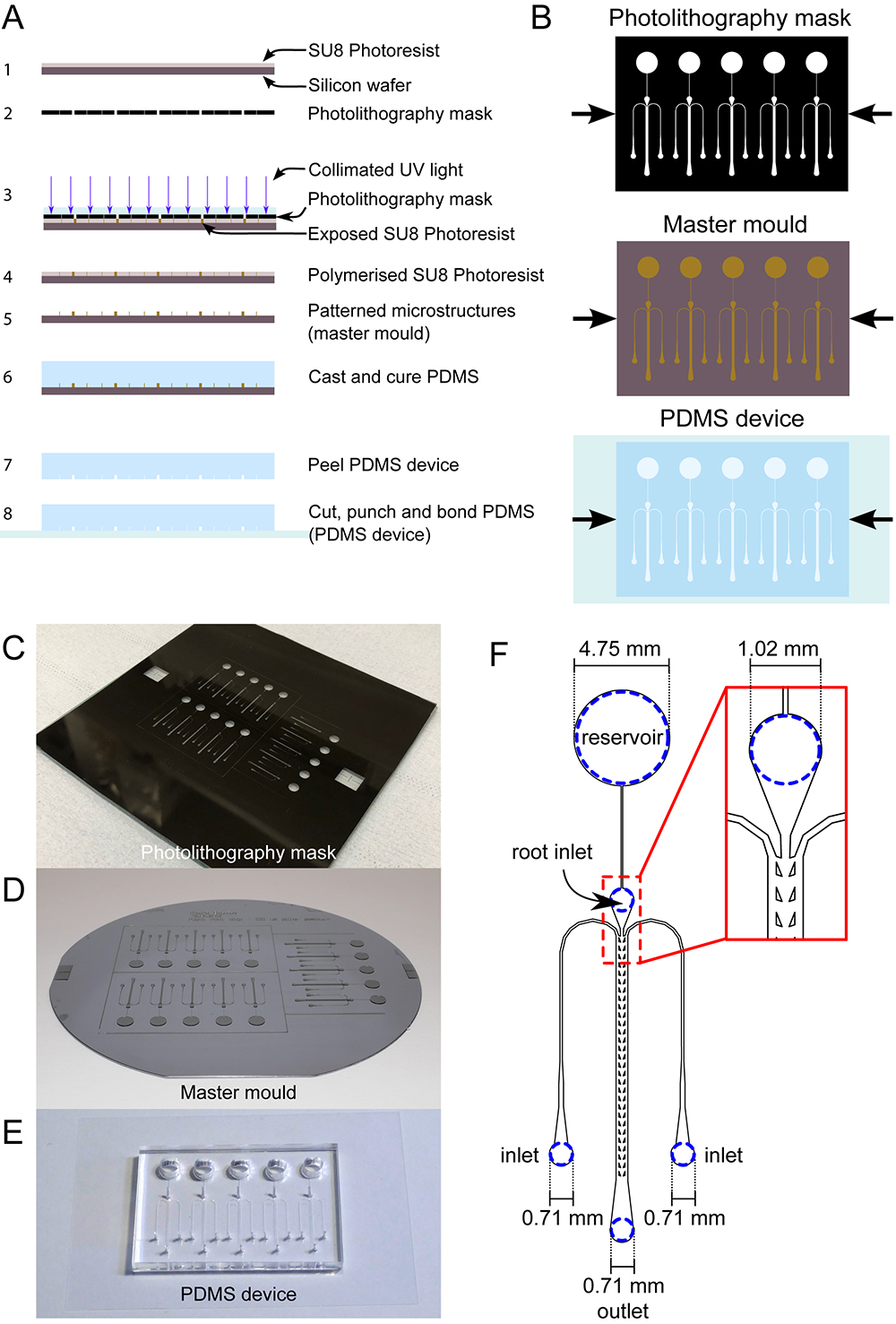
Figure 1. dfRootChip fabrication. A. The photo- and soft lithography processes involved in the fabrication of the dfRootChip are illustrated. First, a silicon wafer is coated with SU8 photoresist (1), after which a photolithography mask (2) is used to expose specific regions of the photoresist to collimated UV light (3). During exposure, the photoresist is polymerized (4) and then developed. Development removes regions of unpolymerised photoresist, resulting in a master mould with patterned microstructures (5). Poly(dimethylsiloxane) (PDMS) is then cast and cured (6), after which it can be removed from the master mould (7) and cut, punched and bonded to a glass coverslip coated with a thin layer of PDMS to form a PDMS device (8). B. To complement the “side-view” illustrations in A, “top-view” illustrations of the photolithography mask, master mould and PDMS device have been included for clarity. C-E. Photographs of an exemplar photolithography mask (mounted on a soda-lime glass plate), a master mould and PDMS device are displayed in C, D and E respectively. F. The specific dimensions and positions of the inlets and outlet are displayed for clarity. - PDMS device fabrication (for detailed video instructions on PDMS device fabrication see Video 1; process outlined in Figures 1 and 2; see Notes 1 and 4)
- Prepare poly(dimethylsiloxane) (PDMS) using a 10:1 ratio of base to curing agent (Sylgard 184 Kit).
- Mix thoroughly using a spatula.
- Degas the mixture using a vacuum pressure of 50 mbar for 1 h.
- Pour the PDMS onto the master mould (see Note 5).
- Coat five clean coverslips (rinsed with acetone, IPA and blow-dried) with a thin layer of PDMS (Laurell Technologies, spin coat at ~1,200 rpm for 25 sec).
- Cure the master mould and coated coverslips overnight in an oven at 70 °C.
- Allow the master mould to cool to room temperature and remove the cured PDMS.
- Cut out the PDMS devices using a utility knife. In the cutting device shown in Figure 2E, the knife blade is fixed in a custom-made metal frame and pushed down to cut cured PDMS slabs.
- Punch holes to create the root inlets (Ø = 1.02 mm), solution in- and outlets (Ø = 0.71 mm) and reservoirs (Ø = 4.75 mm). The root inlets should be punched at ca. 30-45° angle.
- Wash the devices and coverslips in each of the following solutions for 5 min using an ultrasonic bath: 0.5 mM NaOH, 70% EtOH and purified water. Rinse the devices in purified water between each washing step.
- Dry the devices and coverslips with compressed air and place in the oven at 70 °C for 1 h.
- Bond the dried devices to the coverslips using a plasma cleaner (see Note 6). Scotch tape can be used to remove dust particles from the surface of the PDMS before bonding.

Figure 2. PDMS device fabrication. The processes involved in preparing a PDMS device are illustrated (see Procedure B for detailed information). The PDMS is prepared by mixing the base and curing agent together (A) and degassing the mixture (B). The degassed PDMS is then poured on top of the master mould (C) and then removed after curing in an oven (D). After cutting out (E) and punching holes (F) into the PDMS, it is washed (G), dried (H) and bonded to a glass substrate (I-L). For detailed video instructions on PDMS device fabrication see Video 1.Video 1. PDMS device fabrication. The video shows the entire process of PDMS device fabrication outlined in Procedure B and Figure 2 of the protocol. - Preparing dual-flow-RootChip for on-chip plant cultivation (see Note 1)
- After bonding, the devices should be filled immediately with half-strength Hoagland's medium (½x HM, see Recipe 1). To do this, manually pipette ½x HM into each microchannel via the root inlet. The microchannel is filled when the medium exits through the inlets and outlet. Check for air bubbles, and use more medium as necessary to remove air bubbles. Top up the inlets/outlets and reservoirs with ½x HM to ensure that they are completely filled.
- Sterilize the devices using UV light for 30 min with a UV crosslinking device.
- Surface seed sterilization (see Notes 1 and 7)
- Place one small spatula full of Arabidopsis thaliana seeds (about 100) into a 2 ml microcentrifuge tube.
- Fill the microcentrifuge tube with 1 ml of sterile-filtered sodium hypochlorite solution (5%).
- Shake tube by hand to suspend seeds, then place on vortex for 3-5 min on a medium level.
- Place the tube with the seeds into a centrifuge at ca. 100 x g for a few seconds to spin down the seeds. Continue the procedure under sterile conditions (biosafety cabinet).
- Pipette off the supernatant.
- Re-suspend the seeds in 1 ml of autoclaved purified water.
- Place on vortex for 20 sec.
- Repeat Steps D4-D7 until seeds have been washed with water three times.
- Store the seeds in the fridge (4 °C, in purified water) for three days for stratification.
- Seed farm preparation (see Figure 3; see Notes 1 and 7; a video of the seed farm preparation procedure has been published in Grossmann et al., 2012).
Note: The steps in this section should be carried out under sterile conditions (biosafety cabinet).- Prepare medium of choice containing 0.7% plant agar (e.g., see Recipe 2). The medium used in this step is dependent upon the experiment to be conducted.
- Whilst still warm (ca. 50 °C), pour the agar-containing medium into sterile Petri dishes (e.g., 10) and fill autoclaved 0.1-20 μl pipette tips (Gilson DL10ST) with 5 μl of the same medium (ca. 48 tips per seed farm). Allow the medium to cool completely. Petri dishes that are not used in the preparation of seed farms can be sealed with Parafilm® and stored in the fridge for later use.
- Cut the pipette tips to a final length of about 5 mm using a heat sterilized cutting blade.
- Set the pipette tips upright into agar plates (ca. 48 tips per plate).
- Take the previously surface-sterilized Arabidopsis seeds and, using a pipette, place one seed on top of each pipette tip.
- Seal the plates with Parafilm® and place them in a climate chamber. Grow the plants continuously under long day conditions (16 h light at 100 μE m-2 sec-1, 22 °C, 50-70% relative humidity).

Figure 3. Arabidopsis seed farm. A. This photograph illustrates a “seed farm” that accommodates 5-day-old Arabidopsis seedlings germinated on agar-filled, cut pipette tips. The seed farm is created by placing Arabidopsis seeds on top of medium-filled pipette tips maintained in an agar plate. B. Prior to transfer onto the chip, seedlings with the proper root length are selected under a dissecting microscope (marked with blue dots on the bottom of the Petri dish in A). - Plant selection (see Note 1; a video of the selection procedure has been published in Grossmann et al., 2012)
- Take a 5-day-old seed farm (see Note 8) and place under a stereo microscope.
- Adjust the light levels and direction of the Petri dish until the roots are visible.
- Identify 8-10 plants per dfRootChip, where roots are at the same developmental stage. Preferably, the roots have just reached the end of the pipette tip, but have not grown past the opening. Slightly shorter roots can work if all roots in one device are at the same developmental stage.
- Mark selected plants for later identification.
- Transferring plants onto the dual-flow-RootChip (see Notes 1 and 7; a video of the transfer procedure has been published in Grossmann et al., 2012).
Note: These steps require sterile conditions (biosafety cabinet).- Sterilize two pairs of forceps and take a pre-bonded and filled dfRootChip (see Procedure C).
- Using forceps, gently insert the pre-identified pipette tips containing plants into the plant inlets (see Procedure F). To prevent the introduction of air bubbles into the microchannels, first put a small amount of medium over the plant inlets.
- Ensure that the pipette tips are inserted into the plant inlet until they almost touch the bottom of the channel. This will help to ensure consistent root growth into the microchannels.
- After all of the plant inlets are filled, place the dfRootChip into a sterile Petri dish.
- Fill the Petri dish with ca. 15 ml of ½x HM (see Recipe 1).
- Seal the Petri dish with Parafilm® and place in the climate chamber for ca. 3-4 days, or until several root tips are visible in the microchannels.
- Performing symmetric and asymmetric treatments with the dual-flow-RootChip: Exemplified with phosphate treatments (see Figure 4 and Note 1)
- Prepare tubing: for each plant on the dfRootChip prepare two identical lengths of tubing with a luer-lock dosage needle at one end and a metal connector pin at the other (to connect the syringes to the device via the inlets, “inlet” tubing), plus another length of tubing with metal connector pins at one end only (to connect the device outlets to a waste container, “outlet” tubing) (Figure 4C). When choosing the proper inlet tubing length, the required distance for full lateral movement of the chip mounted at the microscope stage needs to be considered.
- Autoclave all tubing, then place in a biosafety cabinet.
- Take a dfRootChip containing Arabidopsis plants (see Procedure G) and place in a biosafety cabinet. Using a magnifying glass or dissection microscope, ensure that the roots have grown into the microchannels and have reached a sufficient length, preferably at the beginning of the channels. Also, make sure that the plant itself appears healthy, with green cotyledons.
- Connect the “outlet” tubings to the dfRootChip outlets via the metal pins.
- Inside the biosafety cabinet, fill sterile syringes with the desired media (see Note 9). To perform experiments with 5 plants for example, 10 syringes will be required. To perform asymmetric treatments, fill half of the syringes with the first medium of interest (e.g., see Recipe 3) and half with a second medium of interest (e.g., see Recipe 4).
- Connect each “inlet” tubing to a syringe using dosage needles. Manually push the medium through each tubing, avoiding air bubbles. In case air-bubbles remain inside the chamber, these will be pushed out as soon as the perfusion is started.
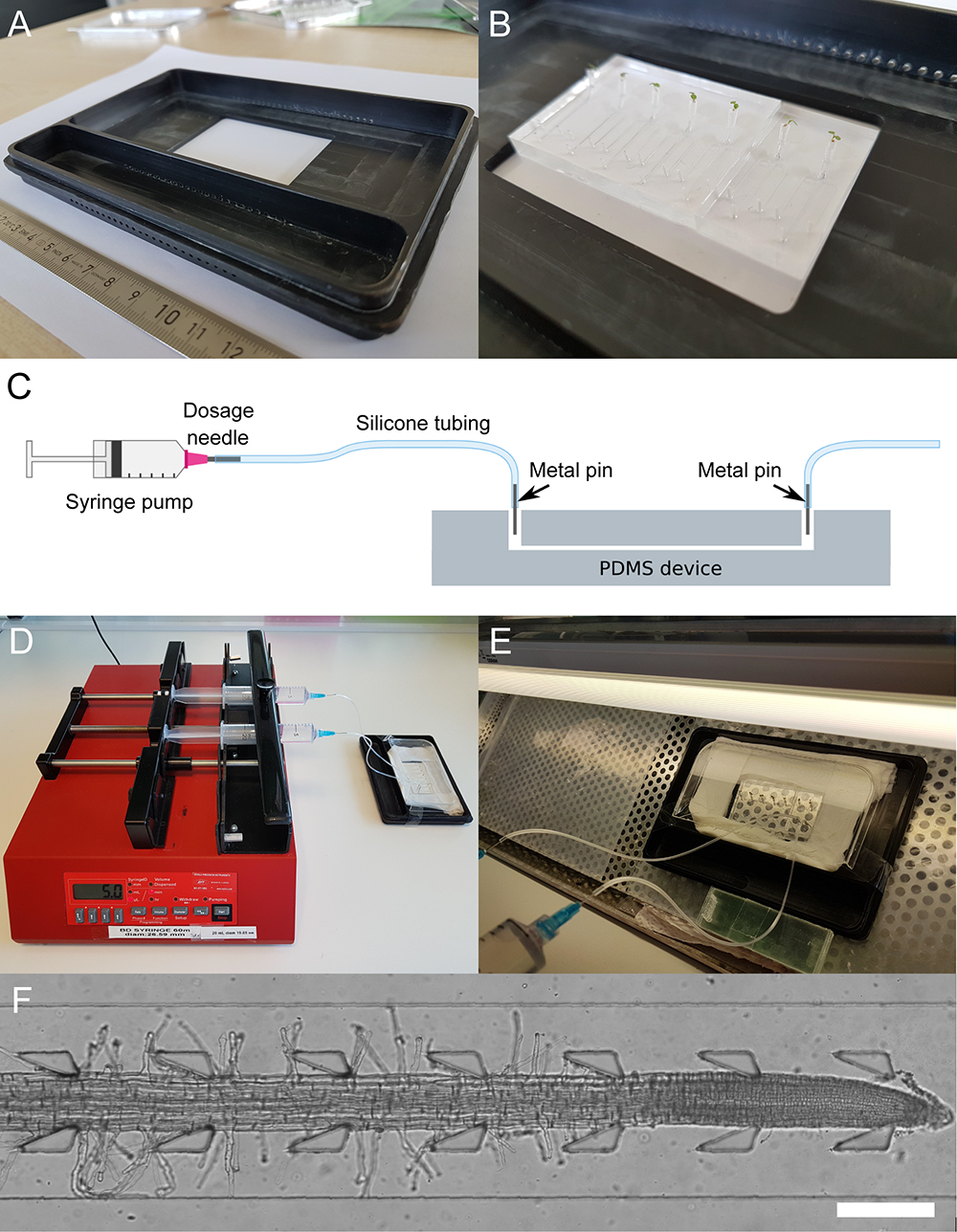
Figure 4. Experimental set-up for performing symmetric and asymmetric treatments with the dfRootChip. A chip holder (A) is used to house the dfRootChip (B) when performing experiments. The dfRootChip is connected to a syringe pump (C-E) to perfuse the roots with the treatment(s) of choice. A cover and wet tissues are used to maintain the humidity around the plants. The set-up can then be transferred to a microscope to image the roots (F, scale bar = 250 μm).- Load the syringes onto the syringe pump, enter the syringe diameter and start perfusion at a high initial flow rate (e.g., 100 μl/min). Note that the syringe pumps can remain outside of the biosafety cabinet.
- Start the pump and leave it to run until the medium is pushed out of the loose tubing ends.
- Set the flow rate to 5 μl/min (Figure 4D).
- While the pump is still running, connect the “inlet” tubings to the inlets via the metal connector pins using sterilized forceps. This ensures that no air bubbles are introduced into the microchannels.
- The dfRootChip is now ready to be imaged. Place the dfRootChip into the chip holder (Figure 4A) and transport the setup to an inverted microscope.
- Localized inoculation of Arabidopsis roots with bacteria trapped by the micropillar array: Exemplified with Pseudomonas fluorescens strain WCS365-GFP (Haney et al., 2015) as proof of concept (see Note 1)
- Culture Pseudomonas fluorescens at 28 °C with shaking (200 rpm) overnight in 100 ml LB medium supplemented with 50 μg/ml kanamycin.
- Check the OD (600 nm) of the bacterial culture. For bacteria to be in log phase, OD should be in the range of 0.1-0.2.
- Transfer the culture into a 20 ml Falcon tube and centrifuge at 12,000 x g for 5 min.
- Discard the supernatant and resuspend the pellet in 20 ml of ½x HM.
- Again centrifuge at 12,000 x g for 5 min.
- Repeat Steps I4 and I5 to remove any traces of LB and kanamycin.
- Gently resuspend the bacterial pellet in ½x HM and use this to fill the syringe(s).
- To inoculate Arabidopsis roots with P. fluorescens, connect the syringe(s) to the dual-flow-RootChip, as detailed in Procedure H.
- Performing rapid asymmetric treatments in the dual-flow-RootChip: Exemplified with a calcium elicitor treatment using 100 mM NaCl solution as proof of concept (see Figure 5 and Note 1)
- Prepare pressurizable vials (screw-neck vials; two per plant on the dfRootChip): Place a septum into the lid of the bottle and puncture it with a metal pin leaving the pin in the septum. Connect a piece of tubing long enough to reach the bottom of the bottle to the inside end of the pin and a length of tubing to the outside end of the pin. Autoclave the bottle and attached tubing. In addition, autoclave, per plant, two extra lengths of inlet tubing with metal pins on one end and one outlet tubing with one pin.
- Place a dfRootChip containing Arabidopsis plants (see Procedure G) in a biosafety cabinet. Ensure that the roots have grown into the microchannels and have reached a sufficient length, preferably at the beginning of the channels (see Step H3).
- Fill one of the sterile vials with a control medium (e.g., see Recipe 1), and another with the treatment medium (e.g., 100 mM NaCl, see Recipe 6).
- Add a luer-lock stopcock valve set (ethanol-sterilized) in a “closed” configuration to the end of the tubing coming from each of the vials. Add the extra lengths of tubing to the stopcock outlets.
- Pressurise the vials by connecting the extra length of tubing to your source of pressurized, clean, dry air and puncture the septum with the pin. Open each of the stopcocks to fill the tubing completely with medium. Once the tubing is filled close the stopcock again.
- Connect the vial containing the control medium into one of the medium inlets and open the stopcock. In our setup, a pressure of 5 PSI results in a volumetric flow rate of 20 μl/min. Initially, the roots are perfused symmetrically, and the control medium will flow out of the medium outlet and the second medium inlet.
- Connect the vial containing the treatment solution into the second medium inlet. The active flow from the control medium will prevent the treatment solution from entering the microfluidic channel.
- When ready to apply a treatment, open the stopcock associated with the treatment vial to start rapid asymmetric perfusion.
- To stop the asymmetric treatment, close the stopcock associated with the treatment vial. Within seconds, the perfusion will return to symmetric control conditions.
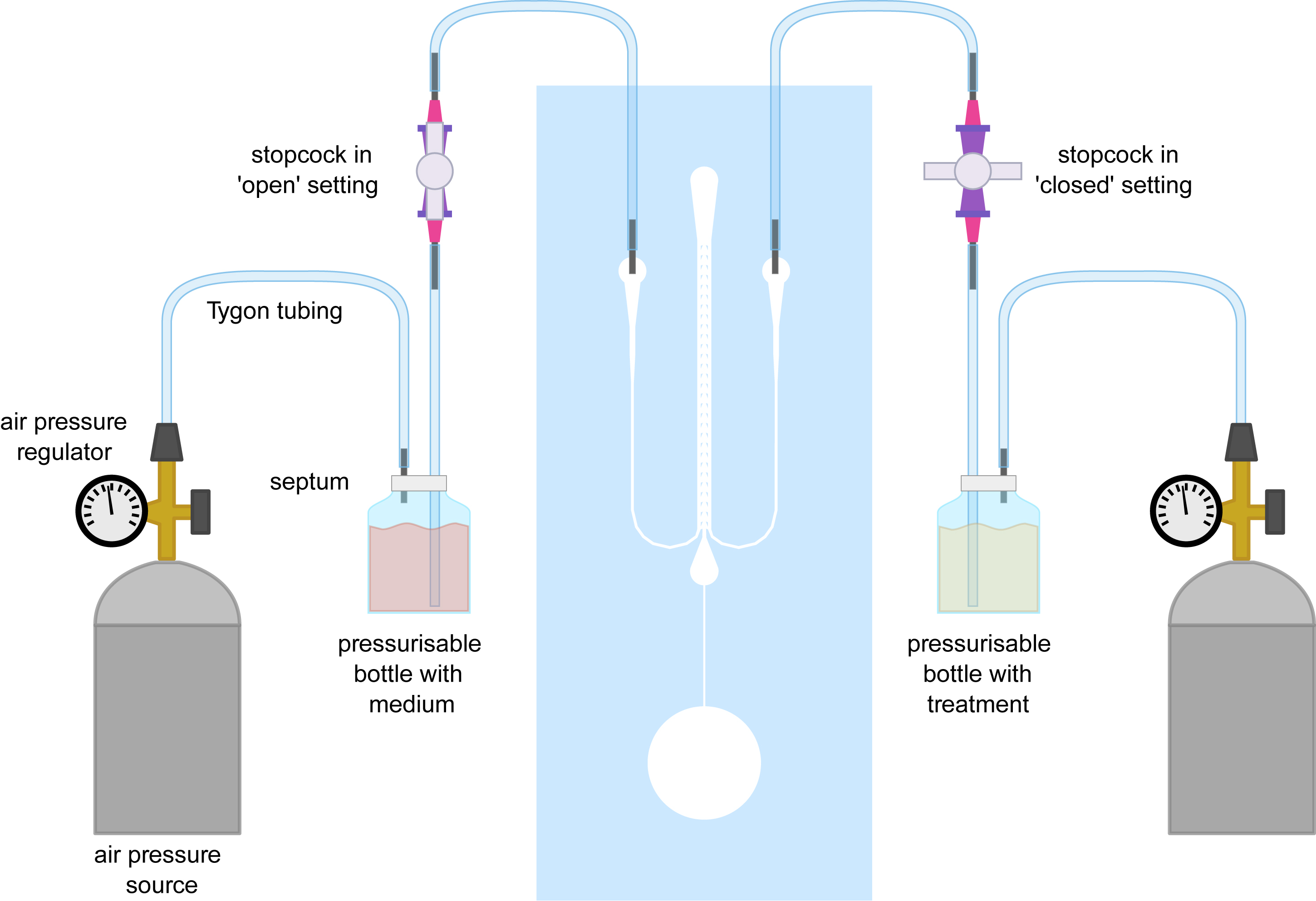
Figure 5. Experimental set-up for performing rapid asymmetric treatments in the dfRootChip. This figure illustrates how the air-pressure sources, pressurizable vials and stopcocks are connected and interfaced with the dfRootChip.
Data analysis
- With regard to experimental design, each dfRootChip enables several experiments (i.e., technical replicates) to be performed in parallel. In addition, a minimum of three biological replicates should be performed for each data set. Examples for analyzing data sets acquired using the dfRootChip can be found in the original article Stanley et al. (2018).
- With regard to data analysis, we recommend using the freehand line tool and kymograph function in Fiji to analyze, for example, primary root and root hair growth rates. To access a wealth of information regarding image analysis, we recommend visiting the following website: http://www.plant-image-analysis.org
Notes
- Please contact us for further information and advice.
- This protocol has been used in our previously published work (Stanley et al., 2018). Parts of the described procedures that are not specific to the use of the dfRootChip are similar to or adapted from previously published work (Grossmann et al., 2011 and 2012; Stanley et al., 2014) but are recapitulated to provide a comprehensive and complete protocol.
- For optimal results, we recommend that the entire fabrication procedure is performed inside a Class 1000 clean room. It is obligatory that the master mould fabrication procedure is conducted under yellow light. We also recommend that all wet chemistry operations are performed in a wet bench with filtered laminar airflow. If clean room facilities are not available, this can be achieved in collaboration with research groups or other commercial services that enable fabrication to be done. Please contact us for further information and advice.
- The channel patterns for the dfRootChip were drawn using AutoCAD Mechanical 2011. The design file can then be printed as polyester film photolithography mask by a commercial provider. The mask layout was arranged such that several dfRootChip replicas could fit in a single 100 mm silicon wafer. Please contact us for further information and advice.
- The processes involved in PDMS device fabrication should be performed in a bench with filtered laminar airflow.
- The master mould can be placed in a 3D-printed plastic holder, as illustrated in Figure 2. Alternatively, aluminum foil can be shaped around a glass Petri dish and used to hold the master mould when pouring PDMS on top of the microstructures.
- When using the plasma cleaner the following conditions were employed: power, 50%; treatment time, 1 min. However, this can vary between products, and the ideal conditions must be determined for each plasma cleaner.
- Work under sterile conditions (e.g., in a biosafety cabinet or next to a flame).
- Growth conditions will vary between laboratories. Therefore, it is important to establish how many days the seeds farms should be incubated in the climate chamber. The plant roots should not grow out of the ends of the pipette tips.
- Use syringes of sufficient size to accommodate specific experimental requirements. As a point of reference, 7.2 ml of medium would be used within 24 h period (per syringe) using a flow rate of 5 μl/min.
Recipes
- ½x Hoagland's medium (½x HM) (1 L)
- Mix 0.8 g of Hoagland’s No. 2 basal salt mixture and 1 g of MES hydrate
- Transfer the above reagents into a 1 L beaker and add 900 ml of purified water
- Dissolve all the above reagents in purified water with the help of magnetic stirrer
- Adjust the pH to 5.7 with KOH
- Add purified water to make the total volume of the medium up to 1,000 ml
- Autoclave the medium for 20 min at 121 °C
- ½x Hoagland's agar medium (1 L)
- ½x HM is prepared as described above (see Recipe 1 steps 1a-1d)
- Weigh 7 g of plant agar (w/v) and add it to the ½x HM
- Make the total volume of the medium up to 1,000 ml with purified water
- Autoclave the medium for 20 min at 121 °C
- Phosphate rich medium (as in Chandrika et al., 2013)
- Mix the following components together to yield these final concentrations in purified water:
2.5 mM KH2PO4
2 mM MgSO4•7H2O
5 mM KNO3
2 mM Ca(NO3)2•4H2O
0.04 mM Na-Fe-EDTA
70 μM H3BO3
0.5 μM CuSO4•5H2O
1 μM ZnSO4•7H2O
14 μM MnCl2•2H2O
0.2 μM Na2MoO4
0.01 μM CoCl2•6H2O
1 g/L MES hydrate - Adjust the pH to 5.5 using 0.5 M KOH
- Sterile filter the medium through a 0.2 μm membrane
- Mix the following components together to yield these final concentrations in purified water:
- Phosphate deficient medium (as in Chandrika et al., 2013)
- Mix the following components together to yield these final concentrations in purified water:
0.01 mM KH2PO4
2.49mM KCl
2 mM MgSO4•7H2O
5 mM KNO3
2 mM Ca(NO3)2•4H2O
0.04 mM Na-Fe-EDTA
70 μM H3BO3
0.5 μM CuSO4•5H2O
1 μM ZnSO4•7H2O
14 μM MnCl2•2H2O
0.2 μM Na2MoO4
0.01 μM CoCl2•6H2O
1 g/L MES hydrate - Adjust the pH to 5.5 using 0.5 M KOH
- Sterile filter the medium through a 0.2 μm membrane
- Mix the following components together to yield these final concentrations in purified water:
- Lysogeny broth (LB) medium (1 L)
- Add 20 g of LB broth mixture and 5 g of NaCl to 1 L of purified water
- Autoclave the medium for 20 min at 121 °C
- 100 mM salt solution (100 ml)
- Prepare ½x HM, as described in Recipe 1
- Dissolve 0.584 g NaCl in 100 ml ½x HM
- Sterile filter the solution through a 0.2 μm membrane
Acknowledgments
This protocol has been adapted from Stanley et al. (2018). We acknowledge financial support from the Swiss National Science Foundation in the form of an Ambizione Career Grant (PZ00P2_168005) to CES, a Faculty for the Future Fellowship by the Schlumberger Foundation to JS, the Deutsche Forschungsgemeinschaft (GR4559/3-1) and research group funds from the Heidelberg Excellence Cluster CellNetworks to GG.
Competing interests
DvS represents Wunderlichips GmbH, Switzerland. All other authors declare no competing interests in regard to this publication.
References
- Busch, W., Moore, B. T., Martsberger, B., Mace, D. L., Twigg, R. W., Jung, J., Pruteanu-Malinici, I., Kennedy, S. J., Fricke, G. K., Clark, R. L., Ohler, U. and Benfey, P. N. (2012). A microfluidic device and computational platform for high-throughput live imaging of gene expression. Nat Methods 9(11): 1101-1106.
- Chandrika, N. N., Sundaravelpandian, K., Yu, S. M. and Schmidt, W. (2013). ALFIN-LIKE 6 is involved in root hair elongation during phosphate deficiency in Arabidopsis. New Phytol 198(3): 709-720.
- Crane, M. M., Chung, K., Stirman, J. and Lu, H. (2010). Microfluidics-enabled phenotyping, imaging, and screening of multicellular organisms. Lab Chip 10(12): 1509-1517.
- Grossmann, G., Guo, W. J., Ehrhardt, D. W., Frommer, W. B., Sit, R. V., Quake, S. R. and Meier, M. (2011). The RootChip: an integrated microfluidic chip for plant science. Plant Cell 23(12): 4234-4240.
- Grossmann, G., Meier, M., Cartwright, H. N., Sosso, D., Quake, S. R., Ehrhardt, D. W. and Frommer, W. B. (2012). Time-lapse fluorescence imaging of Arabidopsis root growth with rapid manipulation of the root environment using the RootChip. J Vis Exp(65): 4290.
- Haney, C.H., Samuel, B. S., Bush, J. and Ausubel, F. M. (2015). Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nature Plants 1: 15051.
- Jiang, H., Xu, Z., Aluru, M. R. and Dong, L. (2014). Plant chip for high-throughput phenotyping of Arabidopsis. Lab Chip 14(7): 1281-1293.
- Keinath, N. F., Waadt, R., Brugman, R., Schroeder, J. I., Grossmann, G., Schumacher, K. and Krebs, M. (2015). Live cell imaging with R-GECO1 sheds light on flg22- and chitin-induced transient [Ca2+]cyt patterns in Arabidopsis. Mol Plant 8(8): 1188-1200.
- Lanquar, V., Grossmann, G., Vinkenborg, J. L., Merkx, M., Thomine, S. and Frommer, W. B. (2014). Dynamic imaging of cytosolic zinc in Arabidopsis roots combining FRET sensors and RootChip technology. New Phytol 202(1): 198-208.
- Massalha, H., Korenblum, E., Malitsky, S., Shapiro, O. H. and Aharoni, A. (2017). Live imaging of root-bacteria interactions in a microfluidics setup. Proc Natl Acad Sci U S A 114(17): 4549-4554.
- Parashar, A. and Pandey, S. (2011). Plant-in-chip: Microfluidic system for studying root growth and pathogenic interactions in Arabidopsis. Applied Physics Letters 98(26): 740.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J. Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P. and Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7): 676-682.
- Stanley, C. E. and van der Heijden, M. G. A. (2017). Microbiome-on-a-Chip: New Frontiers in Plant-Microbiota Research. Trends Microbiol 25(8): 610-613.
- Stanley, C. E., Grossmann, G., i Solvas, X. C. and deMello, A. J. (2016). Soil-on-a-Chip: microfluidic platforms for environmental organismal studies. Lab Chip 16(2): 228-241.
- Stanley, C. E., Shrivastava, J., Brugman, R., Heinzelmann, E., van Swaay, D. and Grossmann, G. (2018). Dual-flow-RootChip reveals local adaptations of roots towards environmental asymmetry at the physiological and genetic levels. New Phytol 217(3): 1357-1369.
- Stanley, C. E., Stockli, M., van Swaay, D., Sabotic, J., Kallio, P. T., Kunzler, M., deMello, A. J. and Aebi, M. (2014). Probing bacterial-fungal interactions at the single cell level. Integr Biol (Camb) 6(10): 935-945.
- Xing, S., Mehlhorn, D. G., Wallmeroth, N., Asseck, L. Y., Kar, R., Voss, A., Denninger, P., Schmidt, V. A., Schwarzlander, M., Stierhof, Y. D., Grossmann, G. and Grefen, C. (2017). Loss of GET pathway orthologs in Arabidopsis thaliana causes root hair growth defects and affects SNARE abundance. Proc Natl Acad Sci U S A 114(8): E1544-E1553.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Stanley, C. E., Shrivastava, J., Brugman, R., Heinzelmann, E., Frajs, V., Bühler, A., van Swaay, D. and Grossmann, G. (2018). Fabrication and Use of the Dual-Flow-RootChip for the Imaging of Arabidopsis Roots in Asymmetric Microenvironments. Bio-protocol 8(18): e3010. DOI: 10.21769/BioProtoc.3010.
Category
Plant Science > Plant physiology > Nutrition
Plant Science > Plant developmental biology > Morphogenesis
Cell Biology > Cell imaging > Microfluidics
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











