- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Increasing the Membrane Permeability of a Fern with DMSO
Published: Vol 8, Iss 12, Jun 20, 2018 DOI: 10.21769/BioProtoc.2896 Views: 5086
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2312 Views

Live-Cell Monitoring of Piecemeal Chloroplast Autophagy
Masanori Izumi [...] Shinya Hagihara
Nov 5, 2025 1688 Views

Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera
Paweł Hermanowicz [...] Justyna Łabuz
Dec 20, 2025 749 Views
Abstract
Cell membrane prevents the entrance of extra molecules (e.g., transcription and translation inhibitors) into the cell. For studying the physiological effects of transcription and translation inhibitors on Hymenophyllum caudiculatum fronds, we incubate fronds with 0.1% DMSO to test if this increases cell membrane permeability relative to incubation with ultrapure water. The study showed that DMSO could significantly improve the cell membrane permeability of filmy fronds.
Keywords: Filmy fernsBackground
One of the most remarkable characteristics of filmy ferns is that the frond, apart from the vascular tissue, is made of a single cell layer and lacks stomata. In our previous work (Garcés et al. 2018), we incubated Hymenophyllum caudiculatum fronds with cycloheximide or actinomycin D to study the effects of translation, or transcription inhibition respectively. If an exogen inhibitor incubated with a filmy fern frond does not affect plant physiology, it may be because the inhibitor fails to enter the cell. In order to improve the entrance of inhibitors into the cell, we tested if an aqueous solution of 0.1% DMSO has effects on cell membrane permeability, visualizing the entrance of propidium iodide (PI) into the cells.
Materials and Reagents
- 1.5 ml microcentrifuge tubes
- Pipette tips
- Plain slides 75 x 25 mm (VWR, catalog number: 48300-026 )
- Micro cover slides, square 22 x 22 mm (VWR, catalog number: 48366-067 )
- Filter (0.22 μm)
- DMSO (Sigma-Aldrich, catalog number: D8418 )
- Propidium iodide (PI) 10 mg (Sigma-Aldrich, catalog number: P4170 )
- Ultrapure water 18.2 MΩcm
- PI (Propidium Iodide) stock (see Recipes)
- PI-H2O control (see Recipes)
- PI-DMSO solution (see Recipes)
Equipment
- Pipettes
- Confocal laser scanning microscope (Olympus, model: Fluoview FV1000 )
- Ultrapure water system (Elga LabWater, model: PureLab Classic )
Software
- FV10ASW v.2.0c
- ImageJ 2.0.0-rc-41/1.50d
Procedure
- Performing the permeability test in 1.5 ml microcentrifuge tubes
- Add 1 ml PI-DMSO solution (Recipe 3) and PI-H2O control (Recipe 2) into different 1.5 ml microcentrifuge tubes. Put freshly obtained, similar size about 1 cm of pinnae into the tubes. Incubate at room temperature.
- After 5 min, 30 min, 2 h, and 5 h take out the pinnae, rinse briefly in distilled water to remove residual PI, and then mount on slides to observe fluorescence under Confocal microscope.
- Add 1 ml PI-DMSO solution (Recipe 3) and PI-H2O control (Recipe 2) into different 1.5 ml microcentrifuge tubes. Put freshly obtained, similar size about 1 cm of pinnae into the tubes. Incubate at room temperature.
- Confocal microscopy
- Observe the Intracellular PI fluorescence under a Fluoview FV1000 confocal laser scanning Biological Microscope (Olympus, Japan). The software FV10ASW v.2.0c and ImageJ 2.0.0-rc-41/1.50d are used to capture the images and construct Z projections. Detect chlorophyll autofluorescence using excitation and emission wavelengths of 488 nm and 687 nm, respectively, PI using excitation and emission wavelengths of 538 and 619 nm, respectively.
- We found that after 30 min of incubation, the PI had entered the cells (Figure 1). Images were obtained from three samples.
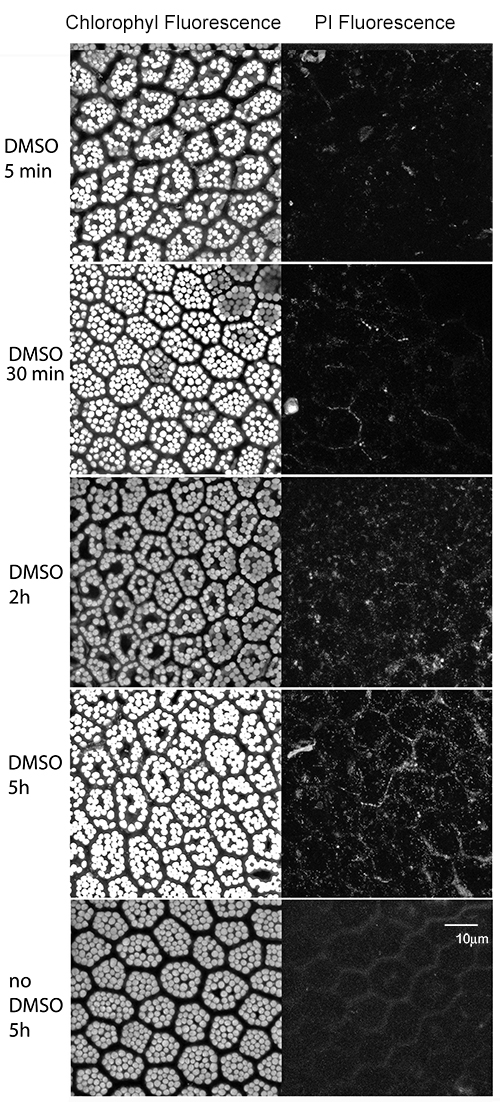
Figure 1. Permeability test for Hymenophyllum caudiculatum fronds. Hymenophyllum caudiculatum fronds were incubated with 0.1% DMSO or distilled water (control) with 1.5 mM PI. Fluorescence of the chlorophyll (left panel) or PI (right panel) is observed. The same field of view was recorded at different wavelengths.
- Observe the Intracellular PI fluorescence under a Fluoview FV1000 confocal laser scanning Biological Microscope (Olympus, Japan). The software FV10ASW v.2.0c and ImageJ 2.0.0-rc-41/1.50d are used to capture the images and construct Z projections. Detect chlorophyll autofluorescence using excitation and emission wavelengths of 488 nm and 687 nm, respectively, PI using excitation and emission wavelengths of 538 and 619 nm, respectively.
Data analysis
Three samples were analyzed and representative results are shown in Figure 1. After 30 min of incubation with 0.1% DMSO, PI fluorescence starts being visible inside the cells and reaches the maximum after 5 h. In contrast, PI fluorescence was almost invisible after 5 h incubation of PI with ultrapure water.
Recipes
- PI (Propidium Iodide) stock solution (1 mg/ml or 1.5 mM)
To prepare, add 10 ml distilled water to 10 mg PI. Store 1 ml aliquots in 1.5 ml tubes at -20 °C, protecting from light - PI-H2O control (10 ml)
1.5 μM propidium Iodide
ultrapure water- In 10 ml of ultrapure water add 10 μl of 1.5 mM PI (stock solution)
- Filter sterilize (0.22 μm) and store at room temperature
- In 10 ml of ultrapure water add 10 μl of 1.5 mM PI (stock solution)
- PI-DMSO solution (10 ml)
0.1% DMSO
1.5 μM propidium Iodide- In 10 ml of ultrapure water add 10 μl of DMSO and 10 μl of 1.5 mM PI (stock solution)
- Filter sterilize (0.22 μm) and store at room temperature
- In 10 ml of ultrapure water add 10 μl of DMSO and 10 μl of 1.5 mM PI (stock solution)
Acknowledgments
This work was supported by grant #11130717 from FONDECYT.
Competing interests
The author declares no conflicts of interest or competing interests.
References
- Garcés, M., Ulloa, M., Miranda, A. and Bravo, L. A. (2018). Physiological and ultrastructural characterisation of a desiccation-tolerant filmy fern, Hymenophyllum caudiculatum: Influence of translational regulation and ABA on recovery. Plant Biol Mar 20(2): 288-295.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Garcés, M. (2018). Increasing the Membrane Permeability of a Fern with DMSO. Bio-protocol 8(12): e2896. DOI: 10.21769/BioProtoc.2896.
Category
Plant Science > Plant physiology > Abiotic stress
Plant Science > Plant cell biology > Cell imaging
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









