- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Method to Injure, Dissect and Image Indirect Flight Muscle of Drosophila
Published: Vol 8, Iss 10, May 20, 2018 DOI: 10.21769/BioProtoc.2860 Views: 9977
Reviewed by: Leonardo Gaston GuilgurThirupugal GovindarajanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Characterization of Biological Motion Using Motion Sensing Superpixels
Felix Y. Zhou [...] Xin Lu
Sep 20, 2019 5934 Views

Live Imaging of Phagoptosis in ex vivo Drosophila Testis
Diana Kanaan [...] Hila Toledano
Mar 20, 2023 1829 Views

Qualitative Detection of Lipid Peroxidation in Mosquito Larvae Using Schiff’s Reaction: A Simple Histochemical Tool for In Situ Assessment of Oxidative Damage
Antonella Cuniolo [...] María Victoria Martin
Feb 5, 2026 54 Views
Abstract
Inducing an injury specifically to Drosophila flight muscles is a difficult task, owing to the small size of the muscles and the presence of the cuticle. The protocol described below provides an easy and reproducible method to induce injury in the Drosophila flight muscles.
Keywords: Drosophila flight musclesBackground
Muscles in vertebrates undergo regeneration, a process attributed to the Satellite cells, the resident stem cells. Our lab has recently shown that Drosophila flight muscles harbor stem cells similar to vertebrate satellite cells, namely insect satellite cells and show proliferation response to muscle injury (Chaturvedi et al., 2017). The ease of fly genetics and our method of inducing injury open up an opportunity to address relevant questions in the field of regenerative biology. We have standardized a protocol for injuring dorsal longitudinal muscles (DLMs) fibers with better precision, and this method can be used for investigating the repair mechanisms involved in muscles after the injury.
Materials and Reagents
- Very thin paint brush
- Minutien pins -Stainless Steel/0.1 mm Diameter (Minutien Pins) (Fine Science Tools, catalog number: 26002-10 )
- Standard fly food media in glass vial (e.g., BDSC Cornmeal Food)
- Standard Petri dish for immunostaining (e.g., FisherbrandTM Petri Dishes with Clear Lid, Thermo Fisher Scientific, catalog number: FB0875713 )
- Adult Drosophila melanogaster
- Cover slips (Thermo ScientificTM Gold SealTM Cover Slips) (Thermo Fisher Scientific, catalog number: 3306 )
- Frosted micro slides, Size: 75 mm long x 25 mm wide, Thickness: 1.35 mm (Blue Star, BLUE STAR (FROSTED MICRO SLIDES))
- Sharp razor blades (e.g., Gillette, 7 O'Clock Super Stainless BladesTM)
- Double sided adhesive tape (Mario Tapes, catalog number: SP 110 )
- Nail polish
- (Optional) Liquid nitrogen
- Ethanol (absolute for analysis EMSURE® ACS, ISO, Reag. Ph Eur) (Merck, catalog number: 1009831011 )
- Sodium chloride (NaCl) (Sodium Chloride, Fisher BioReagents) (Fisher Scientific, catalog number: BP358-1 )
- Potassium chloride (KCl) (Fisher Scientific, Potassium Chloride (Crystalline/USP/FCC), Fisher Chemical, catalog number: P330-500 )
- Sodium phosphate dibasic (Na2HPO4) (Fisher Scientific, Sodium Phosphate Dibasic Anhydrous (USP), Fisher Chemical, catalog number: S375-500 )
- Potassium phosphate monobasic (KH2PO4) (Potassium Phosphate Monobasic (Crystalline/Certified ACS)) (Fisher Scientific, Fisher Chemical, catalog number: P285-500 )
- Triton X-100 (Sigma-Aldrich, catalog number: X100-500ML )
- Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: 05470-5G )
- Phalloidin (1:500 in 1x PBS) (Alexa Fluor 488® phalloidin) (Thermo Fisher Scientific, catalog number: A12379 )
- TOPRO-3-Iodide (1:1,000 in 1x PBS) (TO-PROTM-3 Iodide (642/661) - 1 mM Solution in DMSO) (Thermo Fisher Scientific, catalog number: T3605 )
- VECTASHIELD Antifade Mounting Medium (Vector laboratories, Vectashield®, catalog number: H-1000 )
- 16% Paraformaldehyde (PARAFORMALDEHYDE 16% Aqueous SOL. EM GRADE) (Electron Microscopy Sciences, catalog number: 15710 )
- (Optional) Anti-myosin
- (Optional) DAPI or Hoechst
- 70% (v/v) ethanol (see Recipes)
- 1x Phosphate buffered saline (PBS) (see Recipes)
- 16% Paraformaldehyde (PARAFORMALDEHYDE 16% Aqueous SOL. EM GRADE) (Electron Microscopy Sciences, catalog number: 15710 ) (see Recipes)
- Permeabilization solution (see Recipes)
- Blocking solution (see Recipes)
Equipment
- Stereo microscope (Olympus, model: SZX12 ) equipped with an imaging system (light source: Olympus KL1500 LCD, camera: QIClickTM CCD Camera, model: 01-QICLICK-R-F-CLR-12 , image acquisition software: Micromanager 1.4)
- CO2 pad (e.g., FlyStuff flypad, Genesee Scientific, catalog number: 59-114 )
- A Moria Nickel Plated Pin Holder (Moria Nickel Plated Pin Holder) (Fine Science Tools, catalog number: 26016-12 )
- Forceps (Dumont #5 Forceps) (e.g., Fine Science Tool, catalog number: 11251-10 )
- Scissor (Vannas Spring Scissors–2 mm Cutting Edge) (e.g., Fine Science Tools, catalog number: 15000-03 )
- Laser Scanning Confocal Microscope Olympus FV1000 (FluoView® FV3000 Confocal Laser Scanning Microscope) (e.g., Olympus, model: FV3000 )
- pH meter
Software
- Image acquisition software:
- Olympus Fluoview 1000 for Laser Scanning Confocal Microscope
- Micromanager 1.4 for recording the video
- Olympus Fluoview 1000 for Laser Scanning Confocal Microscope
- Image processing software:
- Fiji
- GNU Image Manipulation Program (GIMP)
- Fiji
Procedure
- Fly muscle injury
- Take one Minutien pin with a pin holder as shown in Figure 1B.
- Clean the pin with 70% v/v ethanol and then with distilled water and dry to avoid contamination. Repeat this procedure every time before pricking the fly with the pin.
- Take 1-2 day old adult Drosophila and anesthetize them with CO2 on a CO2 pad (Figure 1A). Avoid taking more than 10-15 flies on a pad.
Note: Avoid overexposure of CO2 to the flies. Use of cold anesthesia is not suitable for this process as it might have unwanted cold injury effects. This can significantly alter the results. - Place the fly laterally as shown in Figure 1A under a stereo microscope and hold the thorax-abdomen junction gently with the light thin paint brush in one hand.
Note: Avoid use of excessive force while orienting and holding the flies for inducing injury. - While holding the fly, prick the thorax with the pin in PS (presutural) region as circled in Figure 1A. See the Video 1 as a reference.Video 1. Injuring DLMs by a single pinprick at PS (presutural) region. For demonstration purpose, the fly was immobilized using a little drop of nail polish on a glass slide. During actual experiments use of any adhesive is unnecessary. As shown in the video, we make a single prick at PS (presutural) location highlighted with a red arrow (Also shown in Figure 1A). To minimize damage to surrounding tissue, it is crucial to be quick and strictly to avoid moving the pin once it pierces fly thorax. This video was acquired using Stereo microscope coupled with a camera (see Equipment section for technical details). For image acquisition, an open source software micromanager (μManager) has been used. For further reference see Edelstein et al., 2014. Images and videos were processed using Fiji.
- Pricking by the pin should be at an angle of 45° to the A-P axis (anterior-posterior axis is the line running from head to abdomen that divides the animal with bilateral symmetry) so that only DLMs get injured (Figure 1C).
Alternative step: Before pricking the thorax, the pin can be cooled by liquid nitrogen by dipping for a brief moment. - The thorax should be injured with a pin by inserting ~0.5 mm so that only one of the hemithorax get injured (Figure 1D). This will ensure stem cell activation preferably in that hemithorax (Figure 1E) (Chaturvedi et al., 2017).
Note: After the injury, there will be melanization which can be easily identified as a black spot. - Transfer flies into food vial for recovery and process them for immunostaining after 5-6 h of recovery at least (Figures 1D and 1E).
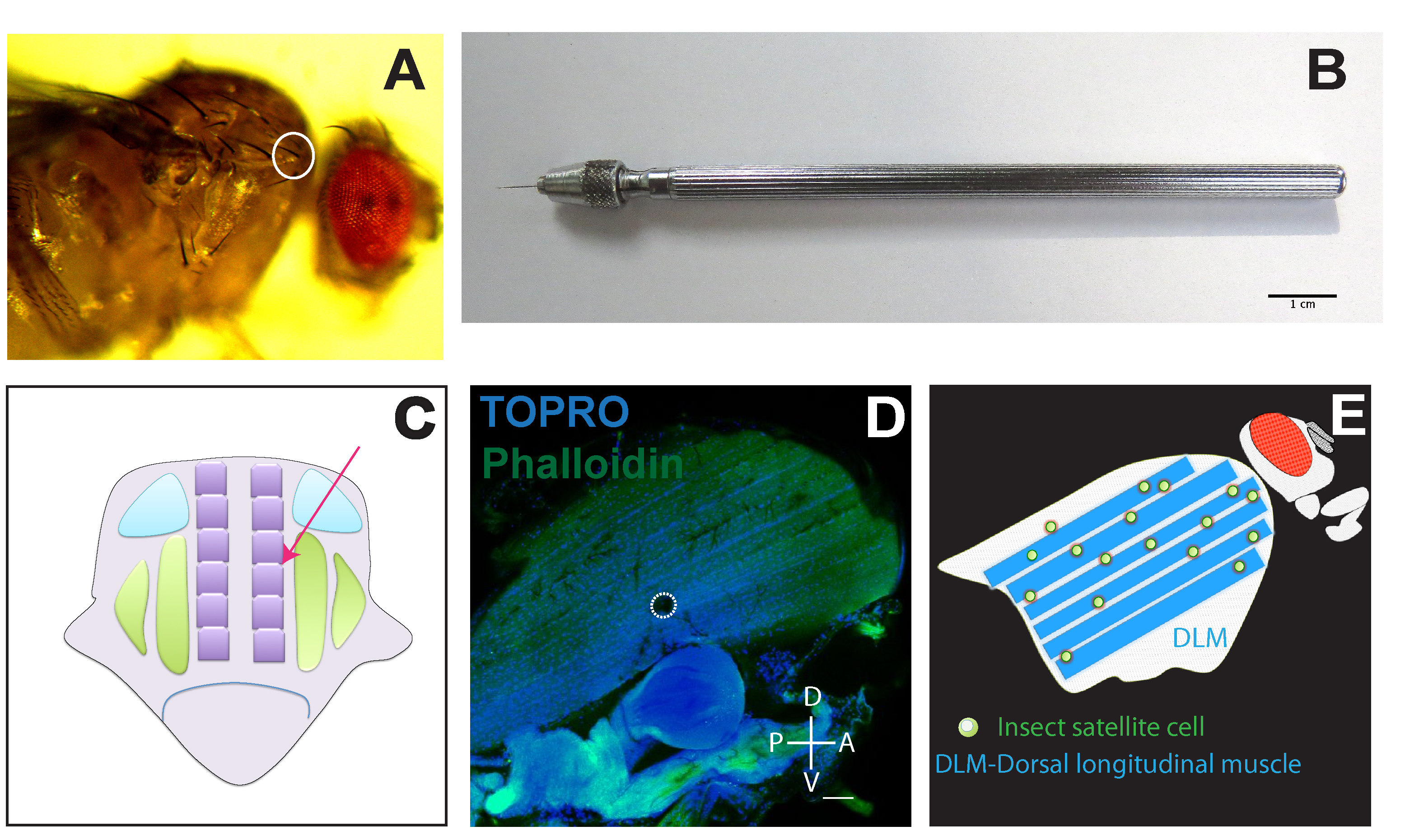
Figure 1. An image guide for inducing injury at Dorsal Longitudinal Muscle. A. Canton-S fly showing the site of injury marked by a white circle; B. A pin with holder for inducing injury, Scale bar = 1 cm. C. Schematic of longitudinal section of fly thorax. The arrow depicts the direction of pin inducing injury to DLM (Dorsal longitudinal muscles, depicted by six purple rectangles). Arrowhead shows the point of contact between a pin and muscle fiber. Green and Blue boxes represent other flight muscles in thorax that are left mostly uninjured. D. Thoracic flight muscles (DLM) showing the site of an injury indicated by a dotted circle (white). Whole mount of flight muscles labeled by phalloidin (green) with all nuclei labeled by TOPRO-3 (blue). Scale bar = 50 µm. E. Simplified scheme depicting unfused muscle stem cells associated with flight muscles.
- Take one Minutien pin with a pin holder as shown in Figure 1B.
- Fly DLM dissection
- Take one adult injured Drosophila and anesthetize.
Note: A semi-alternative method has been given as reference (Weitkunat and Schnorrer, 2014). - Put the anesthetized fly in a petri dish containing 1x PBS (pH 7.5) and use sufficient amount of 1x PBS to keep the animal submerged.
- Hold the fly abdomen with forceps and cut the head, legs, wings, and abdomen with fine scissors under a stereo microscope. Keep only the thoracic part of the fly for rest of the procedure.
Note: To avoid accidental poking into muscles, operate thoracic tissue henceforth using leg stumps or halteres.
- Take one adult injured Drosophila and anesthetize.
- Immunohistochemistry and confocal microscopy
- Submerge the whole thorax in 4% PFA in 1x PBS taken in a Petri dish for chemical fixation for 20 min (see Recipes).
- Take a glass slide and stick double sided tape on the slide.
- Place the thorax on the double-sided tape slide and orient the fly thorax in such a way that the ventral side is up. Then stick it on the tape.
- Make a sagittal cut with a fine razor blade/scissor by following the ventral midline and submerge the hemi-thoraces in 1x PBS solution by holding wings or legs using forceps.
Note: Try to use finely sharpened new blades for better sectioning. - Wash the hemi-thoraces by submerging in permeabilization solution.
- Essentially perform the immunostaining and microscopy as mentioned in Fernandes et al., 1991 and Chaturvedi et al., 2017.
- For staining F-actin, Phalloidin has been used at 1:500 dilution in 1x PBS.
- For staining nuclei, TOPRO-3-Iodide has been used at 1:1,000 dilution in 1x PBS.
- Submerge the whole thorax in 4% PFA in 1x PBS taken in a Petri dish for chemical fixation for 20 min (see Recipes).
- Limitations of the method and expertise required
- If a pin fails to poke through cuticle – The pin has lost its sharpness and replace it with a new pin. A new pin should be used for every set of 20-30 flies for consistent results.
- Diameter of injured area differs between flies – Try a new pin.
- Death of Drosophila post injury – Avoid extensive injury to trachea on the ventral side of the fly.
- Infection/yeast growth in the injured area – Sterilize the pin and holder using 70% ethanol.
- Staining is too faint – Remove excess of tissue such as abdomen, head, and legs.
- Over-fixation of samples can result in absent or low staining. To obtain better results, samples should be fixed only for 20 min using 4% paraformaldehyde (always freshly prepared from 16% stock using 1x PBS).
- Muscle shows the absence of F-actin staining – Muscle injury can lead to areas of low or no phalloidin staining. Alternatively, anti-myosin staining can be used to observe the overall muscle structure.
- The preferred way to stain all the nuclei is to use DAPI/Hoechst along with secondary antibody rather than mounting media with DAPI in it.
- If a pin fails to poke through cuticle – The pin has lost its sharpness and replace it with a new pin. A new pin should be used for every set of 20-30 flies for consistent results.
Data analysis
Image analysis and data processing were essentially performed as mentioned in Chaturvedi et al., 2017.
Recipes
- 70% (v/v) ethanol in ddH2O
- 1x Phosphate buffered saline (PBS) (Cold Spring Harbor Protocols)
NaCl 137 mM
KCl 2.7 mM
Na2HPO4 10 mM
KH2PO4 1.8 mM
Adjust pH to 7.5 - 4% paraformaldehyde for chemical fixation
Make 4% paraformaldehyde from 16% paraformaldehyde in 1x PBS - Permeabilization solution
PBS containing 0.3% Triton X-100 - Blocking solution
PBS containing 0.3% Triton X-100 and 0.1% of bovine serum albumin (BSA)
Acknowledgments
This protocol was adapted from the article, Identification and functional characterization of muscle satellite cells in Drosophila by Chaturvedi et al., 2017. We thank National Centre for Biological Sciences, Tata Institute of Fundamental Research and the J C Bose Fellowship of the Government of India for funding. We acknowledge Central Imaging & Flow Cytometry Facility for using the confocal microscope and NCBS Fly facility. The authors are also thankful to Avishek Ghosh and Rajan Surendra Thakur from Prof. Raghu Padinjat’s laboratory for helping us to use the stereo microscope with a camera attachment. We have no conflict of interest to declare.
References
- Chaturvedi, D., Reichert, H., Gunage, R. D. and VijayRaghavan, K. (2017). Identification and functional characterization of muscle satellite cells in Drosophila. Elife 6.
- Fernandes, J., Bate, M. and Vijayraghavan, K. (1991). Development of the indirect flight muscles of Drosophila. Development 113(1): 67-77.
- Edelstein, A. D., Tsuchida, M. A., Amodaj, N., Pinkard, H., Vale, R. D. and Stuurman, N. (2014). Advanced methods of microscope control using μManager software. J Biol Methods 1(2).
- Weitkunat, M. and Schnorrer, F. (2014). A guide to study Drosophila muscle biology. Methods 68(1): 2-14.
Article Information
Copyright
Chakraborty et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Chakraborty, K., VijayRaghavan, K. and Gunage, R. D. (2018). A Method to Injure, Dissect and Image Indirect Flight Muscle of Drosophila. Bio-protocol 8(10): e2860. DOI: 10.21769/BioProtoc.2860.
- Chaturvedi, D., Reichert, H., Gunage, R. D. and VijayRaghavan, K. (2017). Identification and functional characterization of muscle satellite cells in Drosophila. Elife 6.
Category
Stem Cell > Adult stem cell > Muscle stem cell
Cell Biology > Tissue analysis > Tissue staining
Cell Biology > Tissue analysis > Tissue imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









