- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Intra-amniotic Injection of Mouse Embryos
(*contributed equally to this work) Published: Vol 8, Iss 10, May 20, 2018 DOI: 10.21769/BioProtoc.2854 Views: 7109
Reviewed by: Nolwenn PasquetAnca SavulescuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In situ Microinflammation Detection Using Gold Nanoclusters and a Tissue-clearing Method
Fayrouz Naim [...] Masaaki Murakami
Apr 5, 2023 2670 Views

Mass Spectrometry-based Lipidomics, Lipid Bioenergetics, and Web Tool for Lipid Profiling and Quantification in Human Cells
Liang Cui [...] Kuan Rong Chan
Aug 20, 2023 3005 Views

A One-Step Mouse Model of Parkinson’s Disease Combining rAAV-α-Synuclein and Preformed Fibrils of α-Synuclein
Santhosh Kumar Subramanya [...] Poonam Thakur
Dec 5, 2025 1644 Views
Abstract
Recent outbreaks of infectious neuro-developmental diseases such as congenital Zika syndrome - have led to a demand for prognosis data from animal models. We developed an intra-amniotic injection mice model that allows Zika virus (ZIKV) infected mice to grow to puberty. In this system, ZIKV is injected into the amniotic fluid of pregnant mice and infected embryos thereafter. ZIKV-infected mice show several symptoms of clinical ‘congenital Zika syndrome’, including decreased brain volume and mis-laminated retina. We also evaluated several behavioral functions of these ZIKV-infected mice, for example, after the mice reach puberty, they have visual and motor defects. This technique can be used to screen and evaluate drug candidates and may help evaluate the prognosis of infectious neuro-developmental diseases.
Keywords: Intra-amnioticBackground
It has been more than sixty years since the first human case of ZIKV infection was reported but in 2007 only 14 human cases of ZIKV infection had been recorded. Prognosis data have lagged far behind the recent outbreak of ZIKV in 2015. There is some evidence that mouse models may be effective for prognosis studies because previous reports have proved neurovirulence of ZIKV in mice. Therefore, various methods of ZIKV infection including intravenous injection, intraperitoneal injection, foot-pad injection and brain injection have been used to study congenital Zika syndrome in individuals during the embryonic period or infancy. The intra-amniotic injection model presented here has two advantageous features. First, wild-type mice (C57 BL/6J) can be used and studies are not limited to mice with immunologic deficiencies. Second, ZIKV infected mice can grow into puberty, which is beneficial to studies trying to evaluate prognosis.
Materials and Reagents
- Glass pipettes (World Precision Instrument, catalog number: 4878 )
- Sterile gauze (Winner Medical Group, catalog number: 016935 )
- Cotton ball (Winner Medical Group, catalog number: 50401050 )
- 1 ml Syringe (KDL, catalog number: 60017031 )
- 50 ml centrifuge tube (Corning, catalog number: 430828 )
- Dropper (Shanghai Baiqian Biotechnology, catalog number: J00082 )
- Suture needle (Ningbo Medical Needle Co., LTD, 7/0)
- Pregnant mouse (E15) (Shanghai Yison Biotechnology Company, strain: C57 BL/6J)
- 75% alcohol (Sinopharm Chemical Reagent, catalog number: 80176960 )
- Oxygen (Shanghai Lvmin Gas company, purity > 99%)
- Cyanoacrylate (Pattex®, catalog number: PSK12CT-2 )
- Isoflurane (RWD Life Science, catalog number: R510-22 )
- Iodophor (Shanghai Likang Disinfectant Hi-Tech, catalog number: 310100 )
- Lidocaine (MP Biomedicals, catalog number: 190111 )
- Ampicillin (Inalco, catalog number: 1758-9314 )
- Dulbecco’s modified Eagle’s medium (DMEM) (Corning, catalog number: 10-013-CV )
- 10% fetal bovine serum (FBS) (Biological Industries, catalog number: 04-001-1ACS )
- Pen-Strep Solution (Penicillin: 10,000 U/ml, Streptomycin: 10 mg/ml) (Biological Industries, ISRAEL, catalog number: 03-031-1B )
- Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655 )
- Sodium phosphate dibasic dihydrate (Na2HPO4·2H2O) (Sigma-Aldrich, Fluka, catalog number: 71645 )
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S5886 )
- 0.1 M Phosphate buffer solution (PBS) (see Recipes)
- Ampicillin solution (see Recipes)
- Culture medium (see Recipes)
- 1% lidocaine solution (see Recipes)
Equipment
- Flaming/brown micropipette puller (Sutter Instrument, model: P-97 )
- Anesthesia machine (RWD Life Science, catalog number: R610 )
- Water bath (Jinghong Experimental Equipment, model: XMID-8222 )
- Tweezers (VETUS, catalog number: ST-11 )
- Scissors (RWD Life Science, catalog number: S12003-09 )
- Shaver (Codos, catalog number: KP-3000 )
Procedure
- Sterilization: sterilize all tools that will be used in this surgery by autoclaving at 121 °C, 0.12 MPa or with 75% alcohol.
- Fill two 50 ml centrifuge tubes with PBS and Ampicillin, respectively. Before use, warm these solutions in a 45 °C water bath.
- Pipette preparation: We couple a glass pipette with a 1 ml syringe for injecting a large amount of solution (Figure 1). The diameter of the pipette tip is ~50 µm. If the diameter of pipette tip is too large, amniotic fluid will overflow and this may affect the development of fetus. If the diameter of the pipette tip is too small, the pipette can be easily blocked, which increases the duration of time that the embryos are exposed (Figure 2).

Figure 1. Glass pipette and syringe coupled together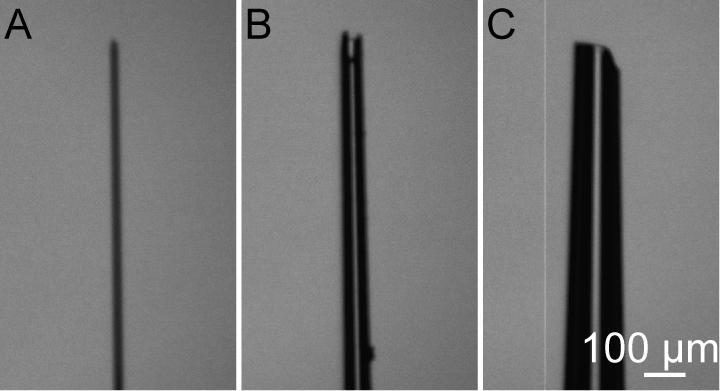
Figure 2. Glass pipette tip with different diameters. A. The tip diameter is too fine. B. The tip diameter is good. C. The tip diameter is too large. - Anesthetize a pregnant mouse (E15) with 1.5% isoflurane in oxygen in an induction chamber at a respiratory rate of 60 times per minute.
Note: If the age of embryos is older than E17 or younger than E14, the survival rate of mice will decrease. - Remove the pregnant mouse from the induction chamber. Place the head of the mouse in a respiratory mask infused with 0.5-1% isoflurane in oxygen. Maintain a respiratory rate between 80 and 100 times per minute to ensure that the embryos do not achieve hypoxia status and that the pregnant mouse is fully anesthetized during surgery.
- Shave the mouse abdomen using a shaver that was sterilized with cotton ball with 75% alcohol and iodophor.
- Drape sterile gauze over the abdomen and make a 2 cm long slit to expose the abdominal incision.
- Cut a 2 cm long incision on the abdominal skin and peritoneum around the position of the womb being careful to cut from outside to inside. Be careful not to damage the capillary vessels on the peritoneum, because bleeding from damaged vessels can lead to wound infection.
- Hold the uterine wall with tweezers and pull out embryos that are in the uterus. Be careful not to damage vessels on the uterine wall especially those that are close to the placenta. Keep the surface of mouse embryos moist using sterilized PBS during the injection process (Figure 3).
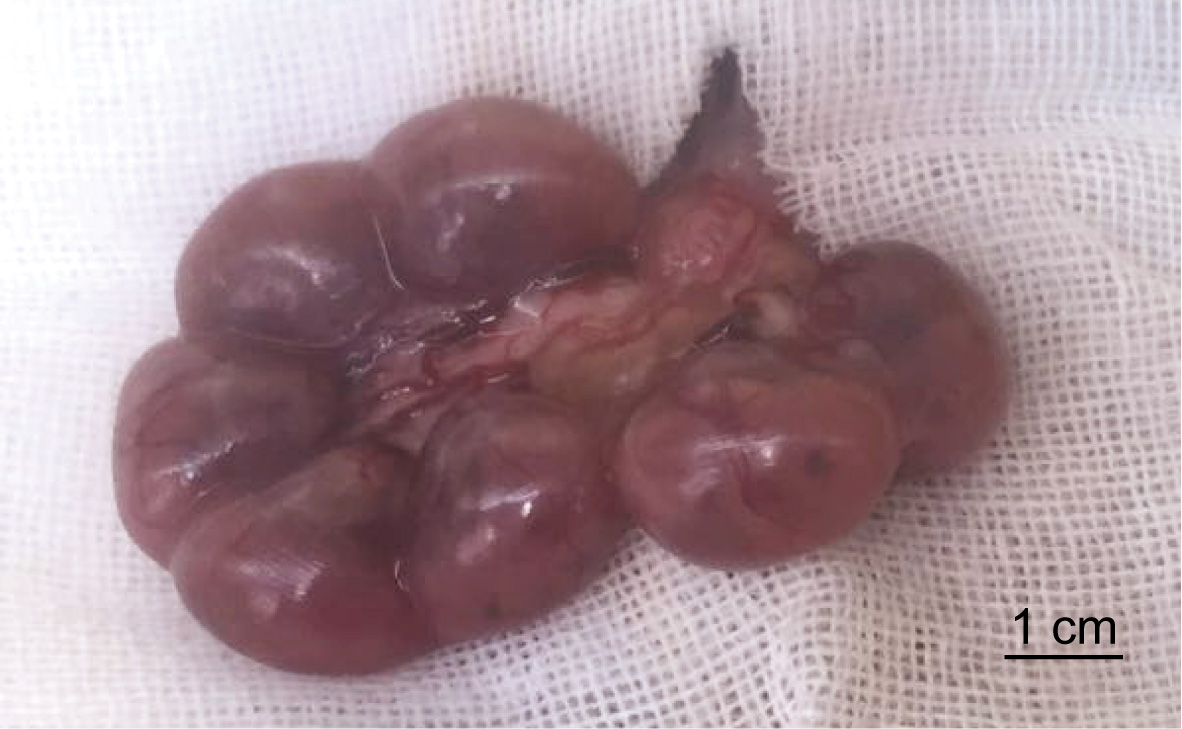
Figure 3. Embryos before injection - With one hand, hold the tweezers and gently lift the uterine wall. With the other hand, hold the injector to inject 0.1 ml culture medium (see Recipes) (injection speed: ~10 µl/sec) into the amniotic at the position close to the tweezers (Video 1).Video 1. The posture of intra-amniotic injection
- Inject the other embryos with a similar volume and injection speed. The survival rate of embryos is typically about 85%.
- Insert the embryos back into the abdominal cavity with tweezers after the injection.
- Fill the abdominal cavity with 2 ml 0.1 mg/ml warm ampicillin in PBS to prevent infection. Remove the extra solution with gauze at the surface of the abdomen.
- Carefully suture the peritoneum and abdominal skin from inside to outside.
- To ease pain, apply 1% lidocaine solution (see Recipes) to the wound on the abdominal skin. The time it takes to complete the surgery (Step 6 to Step 15) should not exceed half an hour. The embryo survival rate decreases if the surgery exceeds 30 min.
Note: The specific operation of Steps 6-15 is shown in Video 2.Video 2. Intra amniotic Injection of Mouse Embryos - Move the pregnant mouse back to her home cage and kept her warm with a heating pad at 37 °C for 3-5 h until it recovers from anesthesia and can move freely.
- ZIKV infected mice show walking defects or paralysis. Videos of ZIKV mice exhibiting this behavior can be found here: http://www.ebiomedicine.com/article/S2352-3964(17)30184-6/addons.
Data analysis
Analysis of behavioral tests including open filed test, tail suspension test, rota-rod test, catwalk and elevated plus maze in the visual and motor deficits of ZIKV infected mice can be found online at http://www.ebiomedicine.com/article/S2352-3964(17)30184-6/fulltext#s0010.
Notes
- The diameter of the suture needle should be about 120 µm. The diameter of a stitch should be about 70 µm. The larger the diameter of the suture needle and stitch, the greater the wound injury will be, especially to the abdominal wound. A fine stitch used to suture will help healing.
- To ensure that the culture medium is injected into the amniotic fluid, you need to inject about 5 µl culture medium near to the sterile gauze before injecting into the amniotic fluid. If the tip is not blocked, you will see culture medium flowing. Similarly, check to ensure the tip is not blocked after you inject into the amniotic fluid.
- If the pregnant mouse keeps lying down on its abdomen, you need to apply 1% lidocaine solution on the wound again and inject 0.3 ml 0.9% sodium chloride solution in the neck to avoid hydropenia.
Recipes
- 0.1 M PBS solution (pH 7.4)
0.02 M potassium phosphate monobasic (KH2PO4)
0.04 M sodium phosphate dibasic (Na2HPO4)
0.1567 M sodium chloride (NaCl) - Ampicillin solution
100 μg ampicillin in 1 ml 0.1 M PBS - Culture medium
DMEM containing:
10% FBS
100 units/ml of penicillin
100 µg/ml of streptomycin - 1% lidocaine solution
1 g lidocaine in 100 ml 0.9% sodium chloride solution
A drop of hydrochloric acid (~40 µl) was added into the solution to help dissolve
Acknowledgments
The authors declare no competing interests. This protocol was adapted from Cui et al. (2017). J.Z. thanks the following funding agencies for supporting this work: the NSF of China (31771195, 81790640), the Young 1000 Plan and Ministry of Science and Technology of the People’s Republic of China (2015AA020512). L. L. thanks the following funding agencies for supporting this work: Ministry of Science and Technology of the People’s Republic of China (2015AA020930 and 2016YFC1202901).
References
- Cui, L., Zou, P., Chen, E., Yao, H., Zheng, H., Wang, Q., Zhu, J. N., Jiang, S., Lu, L. and Zhang, J. (2017). Visual and motor deficits in grown-up mice with congenital Zika virus infection. EBioMedicine 20: 193-201.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Cui, L., Zou, P., Meng, Q., Lu, L. and Zhang, J. (2018). Intra-amniotic Injection of Mouse Embryos. Bio-protocol 8(10): e2854. DOI: 10.21769/BioProtoc.2854.
Category
Cell Biology > Cell Transplantation > Embryo Transplant
Microbiology > Microbe-host interactions > Virus
Neuroscience > Nervous system disorders > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









