- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
FACS-based Glucose Uptake Assay of Mouse Embryonic Fibroblasts and Breast Cancer Cells Using 2-NBDG Probe
Published: Vol 8, Iss 8, Apr 20, 2018 DOI: 10.21769/BioProtoc.2816 Views: 11540
Reviewed by: Aswad KhadilkarChristopher J. PoonAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assessment of Cellular Redox State Using NAD(P)H Fluorescence Intensity and Lifetime
Thomas S. Blacker [...] Gyorgy Szabadkai
Jan 20, 2017 14865 Views

Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism
Birte Plitzko and Sandra Loesgen
May 20, 2018 76079 Views

Optimization of Extracellular Flux Assay to Measure Respiration of Anchorage-independent Tumor Cell Spheroids
Zaineb Javed [...] Nadine Hempel
Feb 20, 2022 4576 Views
Abstract
This is a flow cytometry-based protocol to measure glucose uptake of mouse embryonic fibroblasts (MEFs) and breast cancer cells in vitro. The method is a slightly modified and updated version as previously described (Dong et al., 2017). Briefly, the target cells are incubated with the fluorescently tagged 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG) for 2 h or 30 min, and the efficiency of glucose uptake is examined using a flow cytometer. This method can be adapted to measure a variety of adipocytes, immune cells, MEFs and cancer cells.
Keywords: Mouse embryonic fibroblasts (MEFs)Background
Glucose is the primary source of energy for cells. A family of glucose transporters (GLUT) is responsible for transporting glucose across cell membranes (Kohn et al., 1996). Changes in glucose uptake can reflect the changes in cellular metabolism. For example, tumor cells generally use glucose for aerobic glycolysis in order to support their rapid proliferation. Normally, tumor cells have increased rates of glucose uptake compared to normal cells (Vander Heiden et al., 2009). The 2-deoxyglucose (2DG) is a glucose analog and it accumulates in the cell as 2-deoxyglucose-6-phosphate (2DG6P). 2DG6P has been a gold standard for measuring glucose uptake for a long time (Yamamoto et al., 2011). Although the measurement of radio-labeled 2DG6P is sensitive, many researchers avoid this method because the handling and disposal of radioactive material require a special procedure.
Another non-metabolizable glucose analog is the fluorescently tagged 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxyglucose (2-NBDG). This molecule accumulates in living cells through a glucose transporter and does not enter the glycolytic pathway. Fluorescence generated by 2-NBDG is proportional to glucose uptake. 2-NBDG fluorescence typically displays excitation/emission maxima of ~465/540 nm. It can be detected using optical filters designed for fluorescein using flow cytometry (O'Neil et al., 2005; Zou et al., 2005; Nitin et al., 2009).
Materials and Reagents
- Pipette tips 200 μl tips (USA Scientific, catalog number: 1111-1800 )
- 10 cm Petri dishes (Corning, catalog number: 430167 )
- 15 ml polystyrene centrifuge tubes (Corning, Falcon®, catalog number: 352097 )
- 5 ml round-bottom polystyrene tubes with cell-strainer cap (Corning, Falcon®, catalog number: 352235 ), 0.35 µm nylon mesh
- Embryos of C57BL/6 WT mouse (13.5 days)
- MCF7 breast cancer cells (ATCC, catalog number: HTB-22 )
- Mouse embryonic fibroblasts (MEFs)
- 0.05% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25300062 )
- 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose (2-NBDG) (Thermo Fisher Scientific, InvitrogenTM, catalog number: N13195 )
- Dulbecco’s modified Eagle medium (DMEM), high glucose (GE Healthcare, Hyclone, catalog number: SH30243.01 )
- Fetal bovine serum (FBS) (Gemini Bio-Products, catalog number: 100-106 )
- Penicillin/streptomycin (Pen/Strep) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122 )
- 2-Mercaptoethanol (2-Me) (Sigma-Aldrich, catalog number: M6250-500ML )
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Sodium phosphate dibasic (Na2HPO4)
- Potassium phosphate monobasic (KH2PO4)
- DMEM culture medium (see Recipes)
- Phosphate-buffered saline (PBS, 1x) (see Recipes)
- FACS buffer (see Recipes)
Equipment
- Pipettes (Gilson, model: P200, catalog number: F123601 )
- Refrigerated centrifuge (Eppendorf, model: 5810 R )
- Hemocytometer chamber (Hausser Scientific)
- Water bath (Fisher Scientific, model: IsotempTM 205 )
- Inverted microscope (Nikon Instruments, model: Eclipse Ts2-FL )
- Autoclave
- Cell culture hood (Thermo Fisher Scientific, Forma class II, A2)
- Cell culture incubator (Thermo Fisher Scientific, Forma series II)
- FACSCalibur flow cytometer (BD Biosciences)
Software
- FlowJo software version 10.0.8 or newer (FlowJo)
Procedure
- 2-NBDG uptake assay for MEFs
- Mouse embryonic fibroblasts (MEFs) are isolated from the embryos of C57BL/6 WT mouse (13.5 days) (Dong et al., 2015).
- Culture the MEF cells until reaching 80-90% confluence in 10 cm Petri dishes with DMEM growth medium in a humidified cell culture incubator (37 °C, 5% CO2).
Note: Don’t use MEFs beyond passage 3. MEFs usually become senescent at about passage 4 to 5. - Remove culture medium and wash cells one time with 10 ml 1x PBS.
- Trypsinize cells using 4 ml of 0.05% trypsin-EDTA for 3 min at 37 °C.
- Transfer cells to 15 ml polystyrene centrifuge tubes.
- Harvest cells at 200 x g for 5 min by centrifugation.
- Wash pelleted cells one time with 5 ml 1x PBS.
- Count cells using a hemocytometer chamber.
- Incubate 1 x 106 MEF cells in a 37 °C water bath for 2 h with 1 ml of PBS containing 100 μM 2-NBDG. Incubate the same number of MEFs in the water bath with 1 ml PBS without 2-NBDG as a negative control.
- Pellet the cells at 200 x g for 5 min by centrifugation. After washing the cells with chilled 1x PBS, the cells are pelleted at 200 x g for 5 min by centrifugation.
- Resuspend cells in 0.5 ml of ice-cold 1x PBS with 2% FBS.
Note: Always keep cells on ice after this step. - Filter cells through a 35 µm nylon mesh (the cell-strainer cap of the 5 ml round-bottom polystyrene tubes) to obtain a uniform single-cell suspension in a 5 ml tube.
- Keep the samples on ice until analysis on a flow cytometer.
- Perform flow cytometric analysis on a FACSCalibur flow cytometer. Acquire 10,000 single-cell events per reaction.
- Analyze fluorescence intensity using FlowJo software.
- Mouse embryonic fibroblasts (MEFs) are isolated from the embryos of C57BL/6 WT mouse (13.5 days) (Dong et al., 2015).
- 2-NBDG uptake assay for breast cancer cells
Using the same procedure as MEFs’ uptake assay except incubating 1 x 106 MCF7 cells in a 37 °C water bath only for 30 min (instead of two hours for MEFs) with 1 ml of PBS containing 100 μM 2-NBDG.
Note: Cancer cells increase glycolysis even in the presence of adequate oxygen. To compensate the reduced energy yield due to aerobic glycolysis, there is massive glucose uptake in cancer cells. Glucose transporters (GLUTs) are responsible for constitutive transportation of glucose into cells. Breast tumor and the other tumor cell lines showed higher expression levels of GLUTs 1 to 4 compared to mouse fibroblasts (Maddalena et al., 2015). This is one possible reason why cancer cells have the higher capacity of retaining glucose compared to normal fibroblasts. The other reasons include mitochondria defects and altered PI3K–Akt–mTOR signaling pathway in cancer cells.
Data analysis
True uptake of 2-NBDG-labeled cells is determined using a gating strategy that allows analysis of live cells. 10,000 single cells were collected. We set a gate (R1) on the FSC-SSC plot to select the interested cell population but exclude cell debris. We then displayed the relative fluorescence of the gated cells on the x-axis and the number of cell count on the y-axis. The 2-NBDG positive cells are analyzed by plotting histograms vs. FITC as Figure 1.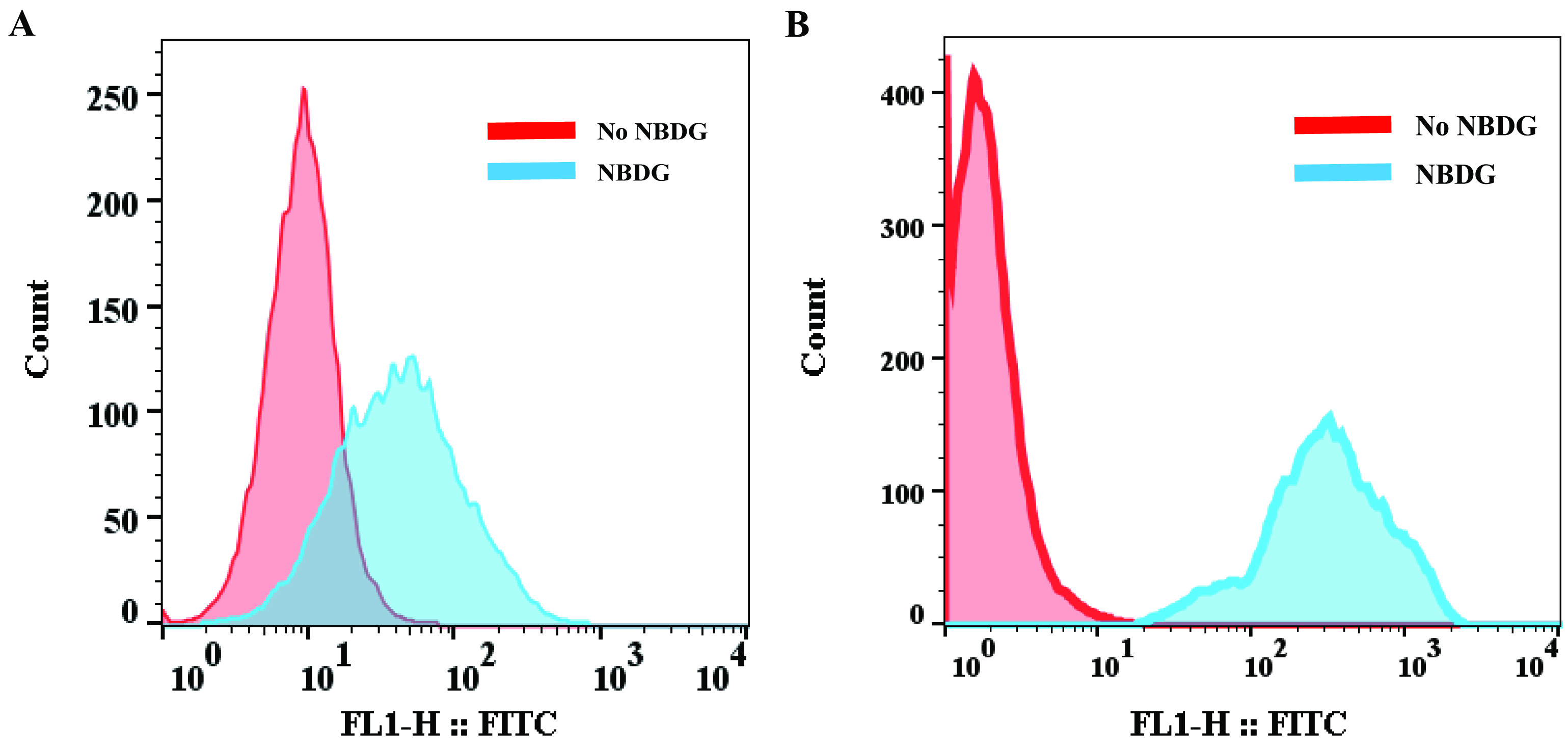
Figure 1. Glucose uptake by MEFs and MCF7 breast cancer cells. A. WT MEFs were assessed for glucose uptake by incorporation of a fluorescent glucose analog 2-NBDG for 2 h. B. MCF7 cells were assessed for glucose uptake by 2-NBDG for 30 min. The fluorescence was detected in the FL-1 (green fluorescence) channel using FACSCalibur and the results are shown as histograms. For quantitation and statistical analysis, we normally calculate the mean value of FL2 fluorescence intensity from triplicate experiments.
Notes
To prevent 2-NBDG leaking from cell membrane, samples should always be kept on ice after stopping the 2-NBDG uptake reaction. Do not keep the samples on ice for more than 2 h before analyzing on the flow cytometer.
Recipes
- DMEM culture medium
DMEM
10% heat-inactivated FBS
1% of penicillin/streptomycin
50 µM 2-mercaptoethanol (2-Me)
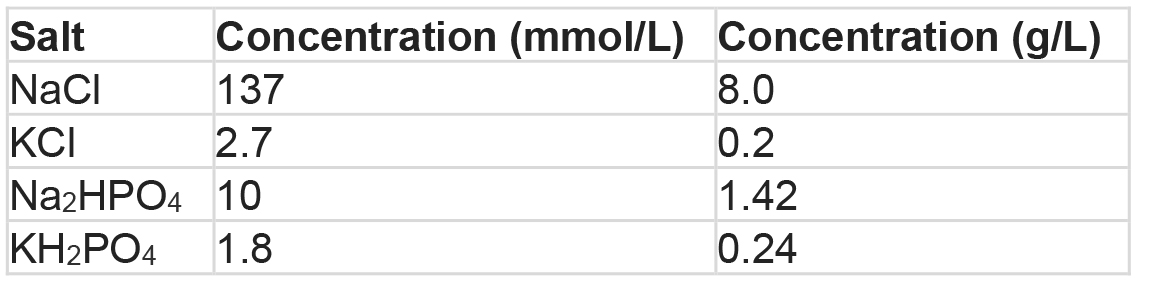
Note: Store at 4 °C; warm to 37 °C before use. - Phosphate-buffered saline (PBS, 1x)
Adjust the pH to 7.4 and then fill distilled water to a final volume of 1 L. Autoclave for 15 min and store at room temperature - FACS buffer
1 x PBS
2% FBS
Note: Prepare just before use and keep on ice.
Acknowledgments
We thank Constance Porretta, who is technical director of Flow Cytometry Shared Resource in Louisiana State University Health Science Center (LSUHSC), for the help with FACS experiments. The authors declare no conflicts of interest. This work was supported by funds from LSUSHC School of Medicine and Fred G, Brazda Foundation. This is an elaborated method based on the work published by Dong et al. (2017).
References
- Dong, S., Baranwal, S., Garcia, A., Serrano-Gomez, S. J., Eastlack, S., Iwakuma, T., Mercante, D., Mauvais-Jarvis, F. and Alahari, S. K. (2017). Nischarin inhibition alters energy metabolism by activating AMP-activated protein kinase. J Biol Chem 292(41): 16833-16846.
- Dong, S., Maziveyi, M. and Alahari, S. K. (2015). Primary tumor and MEF cell isolation to study lung metastasis. J Vis Exp (99): e52609.
- Kohn, A. D., Summers, S. A., Birnbaum, M. J. and Roth, R. A. (1996). Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem 271(49): 31372-31378.
- Maddalena, F., Lettini, G., Gallicchio, R., Sisinni, L., Simeon, V., Nardelli, A., Venetucci, A. A., Storto, G. and Landriscina, M. (2015). Evaluation of glucose uptake in normal and cancer cell lines by positron emission tomography. Mol Imaging 14: 490-8.
- Nitin, N., Carlson, A. L., Muldoon, T., El-Naggar, A. K., Gillenwater, A. and Richards-Kortum, R. (2009). Molecular imaging of glucose uptake in oral neoplasia following topical application of fluorescently labeled deoxy-glucose. Int J Cancer 124(11): 2634-2642.
- O'Neil, R. G., Wu, L. and Mullani, N. (2005). Uptake of a fluorescent deoxyglucose analog (2-NBDG) in tumor cells. Mol Imaging Biol 7(6): 388-392.
- Vander Heiden, M. G., Cantley, L. C. and Thompson, C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930): 1029-1033.
- Yamamoto, N., Ueda, M., Sato, T., Kawasaki, K., Sawada, K., Kawabata, K. and Ashida, H. (2011). UNIT 12.14 Measurement of glucose uptake in cultured cells. In: Enna, S. J. (Ed.) Curr Protoc Pharmacol, Chapter 12, 11-22.
- Zou, C., Wang, Y. and Shen, Z. (2005). 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods 64(3): 207-215.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Dong, S. and Alahari, S. (2018). FACS-based Glucose Uptake Assay of Mouse Embryonic Fibroblasts and Breast Cancer Cells Using 2-NBDG Probe. Bio-protocol 8(8): e2816. DOI: 10.21769/BioProtoc.2816.
- Dong, S., Baranwal, S., Garcia, A., Serrano-Gomez, S. J., Eastlack, S., Iwakuma, T., Mercante, D., Mauvais-Jarvis, F. and Alahari, S. K. (2017). Nischarin inhibition alters energy metabolism by activating AMP-activated protein kinase. J Biol Chem 292(41): 16833-16846.
Category
Cancer Biology > Cellular energetics > Cell biology assays > Metabolism
Biochemistry > Carbohydrate > Glucose
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










