- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assessing Prepulse Inhibition of Startle in Mice
Published: Vol 8, Iss 7, Apr 5, 2018 DOI: 10.21769/BioProtoc.2789 Views: 12124
Reviewed by: Xi FengJuan Facundo Rodriguez AyalaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Surgical Implantation of a Telemetry-Based Pressure Sensor in the Internal Jugular Vein to Monitor Respiration Wirelessly
Neha Kushwaha and Debanjan Dasgupta
Jun 20, 2025 1498 Views

Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1470 Views

Mouse Vestibulo-Ocular Reflex Testing for Otolith Organs and Horizontal Semicircular Canal
Tong Zhao [...] Fangyi Chen
Nov 20, 2025 1199 Views
Abstract
Animal models are an important tool for studying neuropsychiatric disorders. However, a major challenge for researchers working with laboratory rodents is trying to reproduce ‘core’ symptoms of complex human disorders such as schizophrenia. Despite this challenge, however, it is still conceivable to use animal models designed to reproduce some of the disease’s ‘endo-phenotypes’. One example is the prepulse inhibition (PPI) of the startle reflex. PPI is a form of startle plasticity and is characterized by a normal reduction in startle magnitude that occurs when an intense startling stimulus (or pulse) is preceded by a weaker pre-stimulus (or prepulse). The PPI paradigm is commonly used to evaluate sensorimotor gating and it has been described in numerous species including humans and rodents. Deficits in PPI have been observed in subjects with schizophrenia and other neuropsychiatric diseases, as well as in established animal models of these disorders. The PPI paradigm is therefore largely used to explore genetic and neurobiological mechanisms underlying the sensorimotor gating phenotypes found in these disorders. Thus, it is necessary to set up reliable and reproducible protocols to study PPI in mice.
Keywords: Prepulse inhibition of startleBackground
Sensorimotor gating refers to the ability of a sensory event to suppress a motor response (Cryan and Reif, 2012). One form of sensorimotor gating that has been widely studied in humans and rodents is the prepulse inhibition (PPI) of startle. The startle reflex consists of involuntary contractions of whole-body musculature elicited by sufficiently sudden and intense stimuli. Specifically, the acoustic startle response is characterized by an exaggerated flinching response to an unexpected strong auditory stimulus. PPI is a form of startle plasticity and it is characterized by a normal reduction in startle magnitude that occurs when an intense startling stimulus (or pulse) is preceded by a brief, low intensity prestimulus (or prepulse) (Graham, 1975; Hoffman and Ison, 1980). The PPI paradigm is commonly used to evaluate sensorimotor gating and it has been described in numerous species, including humans (Schwarzkopf et al., 1993) and mice (Carter et al., 1999; Frankland et al., 2004). Impaired PPI is observed in schizophrenia (Braff et al., 2001; Swerdlow et al., 2008), as well as other neuropsychiatric disorders including obsessive-compulsive disorder (Ahmari et al., 2012), Tourette’s syndrome (Swerdlow et al., 2001), Huntington’s disease (Swerdlow et al., 1995) and bipolar disorder (Perry et al., 2001). In patients with psychotic disorders, deficits in sensorimotor gating are associated with cognitive fragmentation and psychotic symptoms (Kapur, 2003). As these deficits have been found both in psychotic patients as well as in animal models (Swerdlow and Light, 2016), the PPI paradigm is largely used in the study of neuropsychiatric diseases and has proven a useful tool for studying and characterizing the effects of several anti-psychotics (Xue et al., 2012), and for exploring the mechanisms underlying psychotic-like behaviors (Geyer, 1999; Ouagazzal et al., 2001).
Materials and Reagents
- Mice (C57BL6/N mice purchased from Janvier Labs, Le Genest-Saint-Isle, France)
Note: If pharmacological treatments are applied before PPI performance, the reagents will depend on the control or drug solutions prepared. Depending on the treatments applied prior to the testing, the animals can be housed in either single or collective cages. - 70% ethanol
Equipment
- SR-LAB startle apparatus with digitized electronic output (SR-Lab, San Diego Instruments, catalog number: 2325-0400 ) (Figure 1)
- Digital sound level meter (FLIR Systems, Extech, catalog number: 407730 )
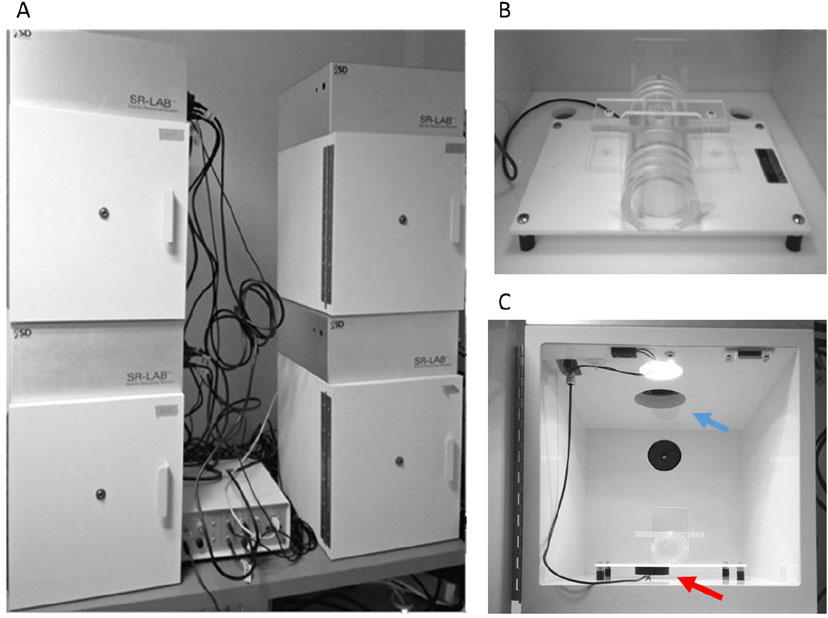
Figure 1. SR-LAB startle apparatus. A. Each experimental apparatus consists of an outer, lighted and ventilated, chamber that serves to prevent external noise or vibrations interfering with experiment. B. Inside the chamber a stabilimeter consisting of a Plexiglas cylinder is secured to a platform. C. A piezoelectric accelerometer-indicated by the red arrow-mounted under the cylinder transduces animal movements that are then digitized, rectified, and recorded by a computer and interface assembly. A loudspeaker-indicated by the blue arrow-generates the startling acoustic stimuli, according to the desired settings.
Software
- SR-Lab Analysis software (SR-Lab San Diego Instruments, catalog number: 2325-0400)
Procedure
- Designing the protocol
Here, we describe the experimental design used in our lab to study PPI response in mice (Busquets-Garcia et al., 2017), but the protocol can be modified by adjusting the pulse and prepulse intensities, the number of trials, inter-trial intervals etc., appropriate for exploring different experimental questions.- Begin the session with a 5-min acclimation period. During the acclimation period, the constant background noise of 70-dB white noise is presented for the animal to adapt to the animal holder, startle box and background noise.
- The session then proceeds through the presentation of 90 different trials (Figure 2):
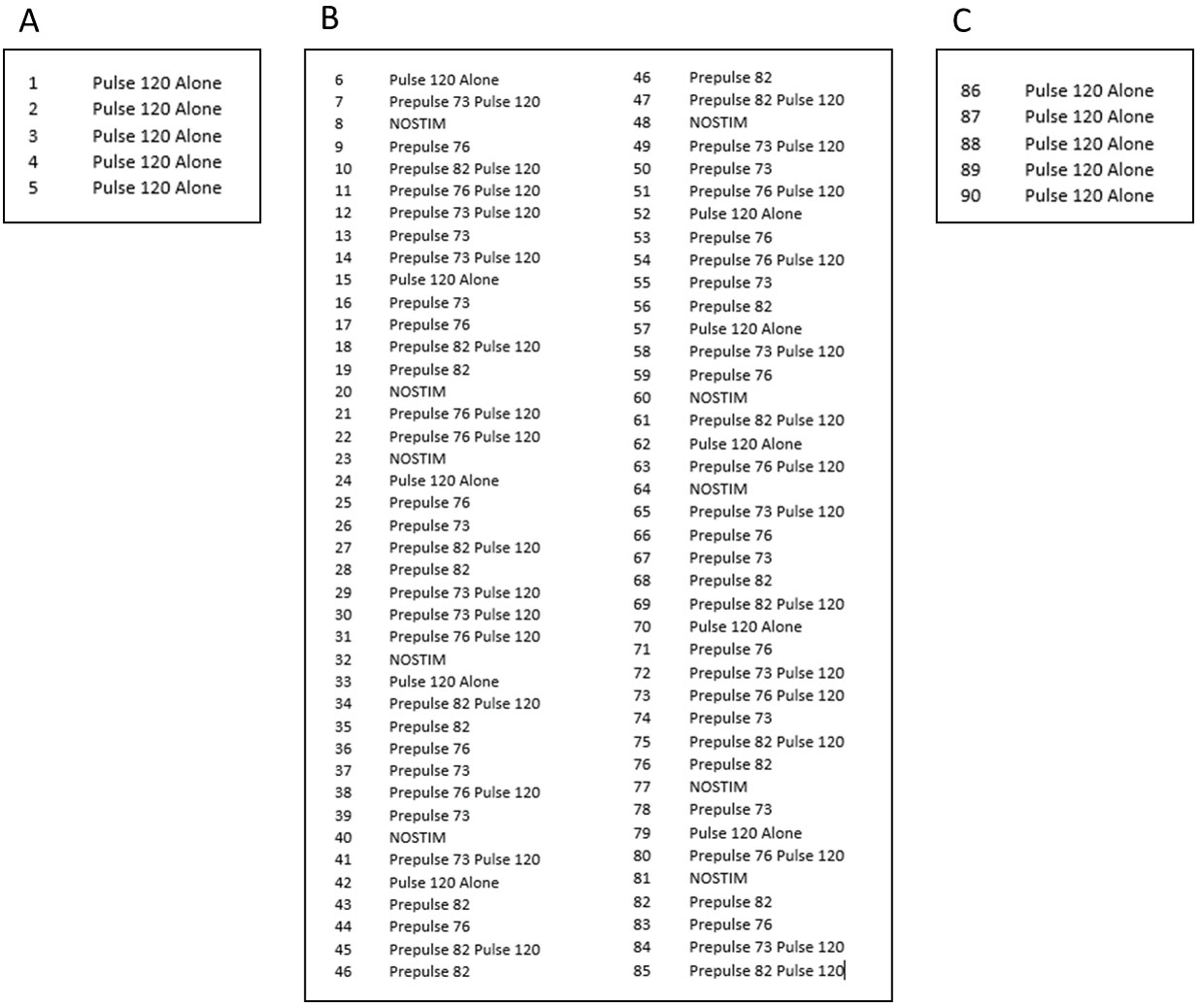
Figure 2. Representative session using the described experimental design. The first five trials consist of five pulse-alone trials (A), the intermediate 80 trials are divided into 10 blocks of randomized pulse-alone trials, prepulse-alone trials, combinations of prepulse-pulse trials and no-stimulus trials (B) and the session is concluded with a final block of five consecutive pulse-alone trials (C). Prepulse intensities (73 dB, 76 dB and 82 dB) are above the 70 dB background.- The first five trials consist of five pulse-alone trials where 120 dB of white noise is presented in isolation for a duration of 20 msec (i.e., with no prepulse). These trials serve to habituate and stabilize the animals to the startle response.
- Subsequently, ten blocks of trials are presented. Each block consists of one pulse-alone trial, three prepulse-alone trials (+3, +6, or +12 units above the background of 70 dB), three combinations of prepulse-pulse trials, and one no-stimulus trial (i.e., background only) (Table 1). The 8 trials are presented in a randomized order within each block, with the inter-trial interval (ITI) varying randomly between 10 and 30 sec, intended to minimize habituation to startle across trials.
Notes:- The advantage of randomized ITIs is in the fact that the animal cannot predict the time when the next stimulus presentation will occur. For example, attention to the prepulse can increase the animal’s efficacy in suppressing startle responses. ITIs below 10 sec should be avoided in order to exclude effects caused by muscle fatigue and refractory periods of muscle responses.
- The intensities of the prepulse should be kept at levels above the background noise but also low enough that they do not elicit a significant startle response on their own, the margin being approximately 2-20 dB above background levels (e.g., +3, +6 or +12 dB above a background of 70 dB). It is important to note that sensitivity to the prepulse may also vary between strain, gender or age of the animals.
Table 1. Representation of the different types of trial used in the behavioral protocol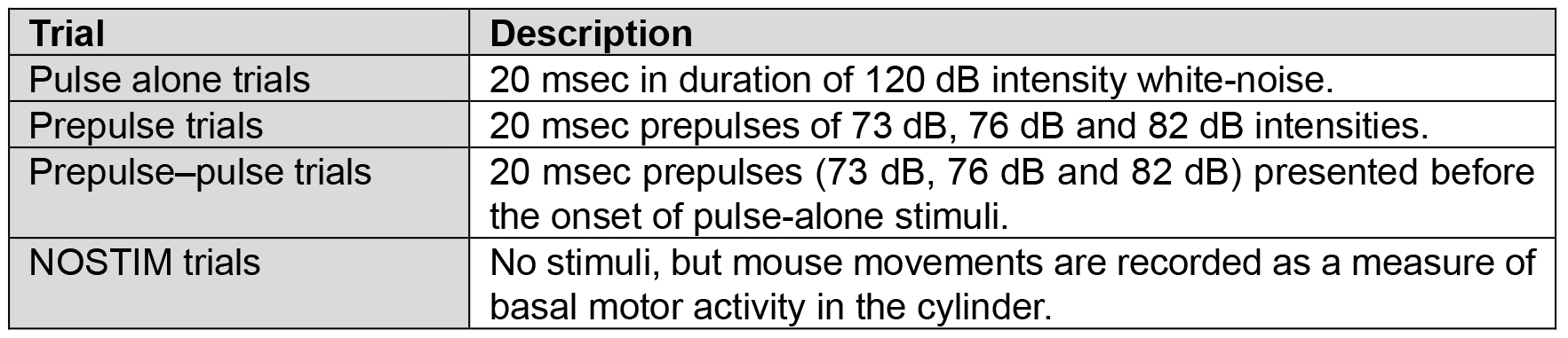
- The advantage of randomized ITIs is in the fact that the animal cannot predict the time when the next stimulus presentation will occur. For example, attention to the prepulse can increase the animal’s efficacy in suppressing startle responses. ITIs below 10 sec should be avoided in order to exclude effects caused by muscle fatigue and refractory periods of muscle responses.
- The session is concluded with a final block of five consecutive pulse-alone trials, as in the first block.
Note: Stimulus rise-time, duration, and intensity are variables that affect startle reflex magnitude (Graham, 1975; Hoffman and Searle, 1968). All the parameters should be carefully decided after taking into account the strain, age, sex and genetic background of the animals, since different lines can exhibit different responses to the startle stimuli (Willott et al., 1995 and 2003).
- The first five trials consist of five pulse-alone trials where 120 dB of white noise is presented in isolation for a duration of 20 msec (i.e., with no prepulse). These trials serve to habituate and stabilize the animals to the startle response.
- Begin the session with a 5-min acclimation period. During the acclimation period, the constant background noise of 70-dB white noise is presented for the animal to adapt to the animal holder, startle box and background noise.
- Running the experiment
- Calibrate the loudspeakers and the sensitivity of the transducer platform of the startle chambers (Figure 3). Follow manufacturer’s guidelines for effective sound and movement calibration.
Note: Calibration of the sound and the movement sensors is very important for obtaining valid test results. Consequently, these must be routinely calibrated before each experiment.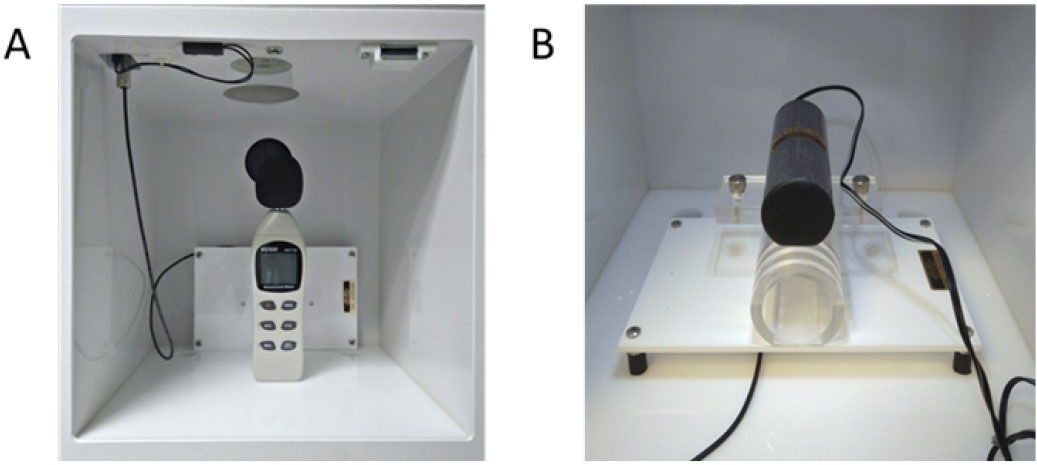
Figure 3. Representative images of loudspeakers (A) and movement sensitivity detector (B) used for the calibration of the startle chambers - Having created your experimental protocol (A1, A2), you can create a study database using the SR-LAB startle apparatus software, defining both the experimental sessions and the subjects that will be tested.
- Transport the mice to the testing room. You can simultaneously test as many mice as the number of chambers you have available (software compatible with up to a maximum of 16 chambers). For housing conditions, see Note 1. Take care not to stress the mice before starting the experiment and, to that end, make no changes to the home cage (e.g., bedding) for at least 24 h before the experiment. Illumination and noise levels in the testing room should be comparable to those in the housing rooms in order to minimize environmental effects on the behavioral outcome. Tubular animal enclosure minimizes stress from being restrained while animal remains centered over the sensor for consistently reliable results.
- In each experimental session, place the mouse in the cylinder inside the testing chamber and secure the door shut.
- Run the experimental session according to the experimental design described above. The session will stop automatically at the end of the protocol (after approximately 35-40 min).
- Remove each mouse from the chamber at the end of the experimental session and return it to its home cage. Wipe clean the animal holders and chambers with water and allow to dry before introducing the next animal.
- Select the next session on the screen and repeat the procedure for all the animals.
- At the end of all the sessions, clean the cylinders and chambers with 70% ethanol and leave them to dry. Save the data obtained for a subsequent detailed analysis of acoustic startle and acoustic prepulse inhibition responses.
- Calibrate the loudspeakers and the sensitivity of the transducer platform of the startle chambers (Figure 3). Follow manufacturer’s guidelines for effective sound and movement calibration.
Data analysis
Reactivity scores obtained on the first and last blocks of five consecutive pulse-alone trials can be analyzed separately to evaluate startle habituation. The data obtained from the remaining 80 trials are categorized into three different subsets according to their relevance to distinct behavioral constructs.
First, startle reactivity (S) is assessed from the reactivity scores obtained in the pulse-alone trials (excluding the first and last blocks of five consecutive pulse-alone trials). The 100 millisecond response window after the presentation of the 120 dB pulse is analyzed by the software and the maximal response peak amplitude is used to determine the acoustic startle response as a control index for the animal’s reaction to the startle pulse (Figure 4A).
Second, reactivity on prepulse-pulse trials (PPiS) relative to the pulse-alone/startle trials (S) is used to evaluate prepulse inhibition (PPI) (Figure 4B). The amount of prepulse inhibition is calculated as a percentage score for each acoustic prepulse trial type using the following formula:
% PPI = 100 x (S - PPiS)/S (Figure 4B)
Third, to measure prepulse-elicited reactivity (PP), data from prepulse-alone trials are included.
The output of a typical experiment should show increasing PPI levels with increasing prepulse intensities, with relatively low variability (Figure 4C). Well-established effects of particular drugs on PPI should be reproducible when using the same strain, sex, and drug dosages. Startle response data typically show more variability and less reliability than PPI response data.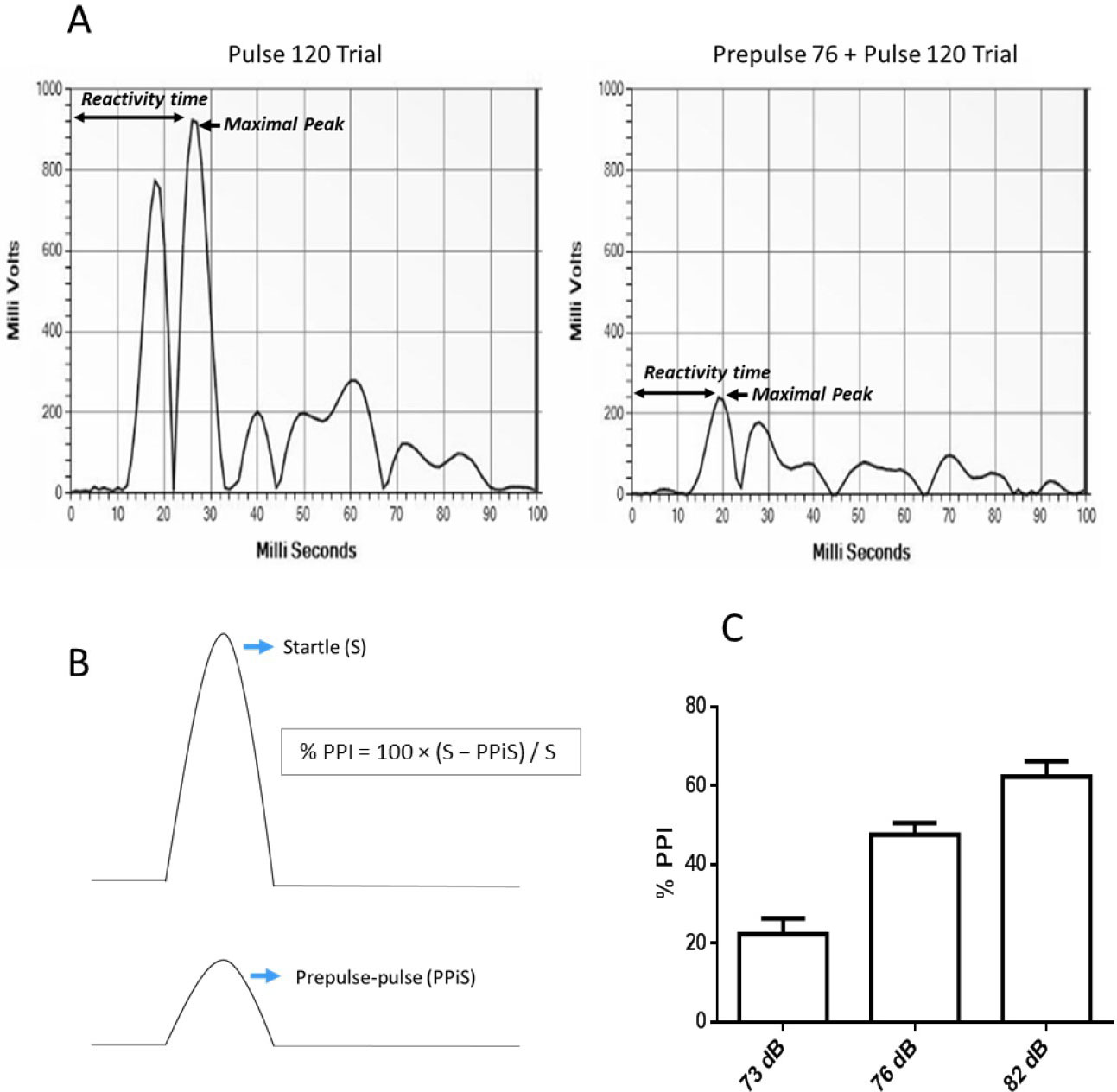
Figure 4. Expected results of a typical PPI experiment. A. Software output of a 100 millisecond response window after the presentation of a pulse-alone and a prepulse-pulse trial. The arrows indicate the maximal response peak amplitude, which is used to determine the acoustic startle response and the maximal response time as an index of the animal’s reactivity to the stimuli. B. Reactivity on prepulse-pulse trials (PPiS) relative to pulse-alone/startle trials (S) is utilized to evaluate prepulse inhibition (PPI). C. Percent prepulse inhibition (%PPI) is shown for naïve wild-type mice with a BL6N background produced in our institute. As expected, PPI levels increase with increasing prepulse intensity (above background), and variability is low.
Notes
- In all PPI experiments performed in our institute, animals were housed in collective cages of 4-5 animals per cage. While social isolation can alter startle variables in rats, the effects of isolation on startle measures in mice have not been well characterized. Therefore, the use of mice that have been separated and single-housed in startle experiments is not ideal.
- All sessions of the experimental protocol were performed in the morning (from 9 AM to 2 PM).
- Strain: Strain differences in startle reflex and PPI have been described in mice (Bullock et al., 1997; Tarantino et al., 2000; Willott et al., 2003). This protocol was tested in several mouse lines. We found similar results between the C57/BL6N mice and the wild-type animals with a BL6N background produced in our institute, although each batch of mice can vary slightly in their responses.
- Sex: Both male and female mice have been used in the protocol of startle reactivity and PPI. If used on the same experimental day, the cleaning process of the testing chambers must be performed meticulously in order to avoid effects on the behavioral outcome.
- Age: Age is an important variable in measures of acoustic startle and PPI. Age-related hearing loss, which can alter startle reactivity and PPI levels, has been reported for many inbred strains including the C57BL/6 (Willott et al., 1995). Moreover, studies have shown that early adolescent mice can display altered startle reactivity and PPI compared to adult animals (Pietropaolo and Crusio, 2009).
- Different psychotogenic drugs (e.g., cannabinoids, amphetamine etc.) can be used as positive controls (i.e., PPI alteration) for the test.
Recipes
The current protocol does not contain any recipes. In case any psychotogenic or other drug is used as a positive control for the test, the preparation should be done according to relevant protocols and manufacturer’s guidelines.
Acknowledgments
We thank Delphine Gonzales, Nathalie Aubailly, and all the personnel of the Animal Facility of the Neurocentre Magendie for mouse care. We also thank Christopher Stevens for the critical reading of the manuscript and Dr. Susanna Pietropaolo for the advice when the PPI was set up in our lab. This behavioral protocol was originally used in Busquets-Garcia et al., 2017. This work was supported by INSERM (to G.M.), EU–FP7 (PAINCAGE, HEALTH-603191 to G.M., FP7-PEOPLE-2013-IEF-623638 to A.B.-G., European Research Council (Endofood, ERC–2010–StG–260515; CannaPreg, ERC-2014-PoC-640923, to G.M.), Fondation pour la Recherche Medicale (DRM20101220445 and DPP20151033974, to G.M.), Human Frontiers Science Program (to G.M.), Region Aquitaine (to G.M.), French State/Agence Nationale de la Recherche (BRAIN ANR-10-LABX-0043 to G.M., ANR-10-IDEX-03-02 to A.B.-G, NeuroNutriSens ANR-13-BSV4-0006-02 to G.M. and ORUPS ANR-16-CE37-0010 to G.M.). The authors declare that they have no conflict of interest.
References
- Ahmari, S. E., Risbrough, V. B., Geyer, M. A. and Simpson, H. B. (2012). Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology 37(5): 1216-1223.
- Braff, D. L., Geyer, M. A., Light, G. A., Sprock, J., Perry, W., Cadenhead, K. S. and Swerdlow, N. R. (2001). Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res 49(1-2): 171-178.
- Bullock, A. E., Slobe, B. S., Vazquez, V. and Collins, A. C. (1997). Inbred mouse strains differ in the regulation of startle and prepulse inhibition of the startle response. Behav Neurosci 111(6): 1353-1360.
- Busquets-Garcia, A., Soria-Gomez, E., Redon, B., Mackenbach, Y., Vallee, M., Chaouloff, F., Varilh, M., Ferreira, G., Piazza, P. V. and Marsicano, G. (2017). Pregnenolone blocks cannabinoid-induced acute psychotic-like states in mice. Mol Psychiatry 22(11): 1594-1603.
- Carter, R. J., Lione, L. A., Humby, T., Mangiarini, L., Mahal, A., Bates, G. P., Dunnett, S. B. and Morton, A. J. (1999). Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci 19(8): 3248-3257.
- Cryan, F. J. and Reif, A. (2012). Behavioral neurogenetics. Springer.
- Frankland, P. W., Wang, Y., Rosner, B., Shimizu, T., Balleine, B. W., Dykens, E. M., Ornitz, E. M. and Silva, A. J. (2004). Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry 9(4): 417-425.
- Geyer, M. A. (1999). Assessing prepulse inhibition of startle in wild-type and knockout mice. Psychopharmacology (Berl) 147(1): 11-13.
- Graham, F. K. (1975). Presidential Address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology 12(3): 238-248.
- Hoffman, H. S. and Ison, J. R. (1980). Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev 87(2): 175-189.
- Hoffman, H. S. and Searle, J. L. (1968). Acoustic and temporal factors in the evocation of startle. J Acoust Soc Am 43(2): 269-82.
- Kapur, S. (2003). Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 160(1): 13-23.
- Ouagazzal, A. M., Jenck, F. and Moreau, J. L. (2001). Drug-induced potentiation of prepulse inhibition of acoustic startle reflex in mice: a model for detecting antipsychotic activity? Psychopharmacology (Berl) 156(2-3): 273-283.
- Perry, W., Minassian, A., Feifel, D. and Braff, D. L. (2001). Sensorimotor gating deficits in bipolar disorder patients with acute psychotic mania. Biol Psychiatry 50(6): 418-424.
- Pietropaolo, S. and Crusio W. E. (2009). Strain-dependent changes in acoustic startle response and its plasticity across adolescence in mice. Behav Genet 39(6): 623-31.
- Schwarzkopf, S. B., Lamberti, J. S. and Smith, D. A. (1993). Concurrent assessment of acoustic startle and auditory P50 evoked potential measures of sensory inhibition. Biol Psychiatry 33(11-12): 815-828.
- Swerdlow, N. R., Karban, B., Ploum, Y., Sharp, R., Geyer, M. A. and Eastvold, A. (2001). Tactile prepuff inhibition of startle in children with Tourette's syndrome: in search of an "fMRI-friendly" startle paradigm. Biol Psychiatry 50(8): 578-585.
- Swerdlow, N. R. and Light, G. A. (2016). Animal models of deficient sensorimotor gating in schizophrenia: are they still relevant? Curr Top Behav Neurosci 28: 305-325.
- Swerdlow, N. R., Paulsen, J., Braff, D. L., Butters, N., Geyer, M. A. and Swenson, M. R. (1995). Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington's disease. J Neurol Neurosurg Psychiatry 58(2): 192-200.
- Swerdlow, N. R., Weber, M., Qu, Y., Light, G. A. and Braff, D. L. (2008). Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 199(3): 331-388.
- Tarantino, L. M., Gould, T. J., Druhan, J. P. and Bucan, M. (2000). Behavior and mutagenesis screens: the importance of baseline analysis of inbred strains. Mamm Genome 11(7): 555-564.
- Willott, J. F., Erway, L. C., Archer, J. R. and Harrison, D. E. (1995). Genetics of age-related hearing loss in mice. II. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear Res 88(1-2): 143-155.
- Willott, J. F., Tanner, L., O'Steen, J., Johnson, K. R., Bogue, M. A. and Gagnon, L. (2003). Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behav Neurosci 117(4): 716-727.
- Xue, Y. Y., Wang, H. N., Xue, F. and Tan, Q. R. (2012). Atypical antipsychotics do not reverse prepulse inhibition deficits in acutely psychotic schizophrenia. J Int Med Res 40(4): 1467-1475.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Ioannidou, C., Marsicano, G. and Busquets-Garcia, A. (2018). Assessing Prepulse Inhibition of Startle in Mice. Bio-protocol 8(7): e2789. DOI: 10.21769/BioProtoc.2789.
Category
Neuroscience > Behavioral neuroscience > Sensorimotor response
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








