- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Fluorescent Dye Method Suitable for Visualization of One or More Rat Whiskers
(*contributed equally to this work) Published: Vol 8, Iss 5, Mar 5, 2018 DOI: 10.21769/BioProtoc.2749 Views: 6969
Reviewed by: Soyun KimEmmanuelle BerretAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Conditioned Lick Suppression: Assessing Contextual, Cued, and Context-cue Compound Fear Responses Independently of Locomotor Activity in Mice
Youcef Bouchekioua [...] Yu Ohmura
Dec 5, 2022 1641 Views

Developing a Ministroke Model in Mouse Barrel Cortex
Song Wang [...] Yihan Wu
Mar 20, 2025 2313 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2571 Views
Abstract
Visualization and tracking of the facial whiskers is critical to many studies of rodent behavior. High-speed videography is the most robust methodology for characterizing whisker kinematics, but whisker visualization is challenging due to the low contrast of the whisker against its background. Recently, we showed that fluorescent dye(s) can be applied to enhance visualization and tracking of whisker(s) (Rigosa et al., 2017), and this protocol provides additional details on the technique.
Keywords: WhiskerBackground
Over the last 10 years, neuroscientists have begun to focus on sensorimotor processing in behaving rodents. A critical step is to be able to quantify the signals that enter the nervous system from the outside world. For whisker-mediated touch, visualization and tracking of the facial whiskers are required in order to characterize the sensory input. Though many approaches have been employed, only high-speed videography has proven adequate for measuring whisker motion and deformation during interaction with an object. However, whisker visualization and tracking is challenging for multiple reasons, primary among them the low contrast of the whisker against its background. This protocol details a technique for increasing contrast by rendering whiskers fluorescent.
Materials and Reagents
- Latex gloves (DOCzero)
- Face mask (Benefis)
- Eppendorf PCR tube (2 ml)
- Cotton pads or gauzes (Megapharma)
- Cotton swabs (DaklaPack)
- Laboratory film (Parafilm)
- Borosilicate glass capillary inside Ø 0.58 mm (Hilgenberg) of 5 cm length
- Syringe (Nipro Europe N.V.)
- Standard aluminum foil
- Animals: Wistar rats (450-550 g) from Envigo-Harlan
Notes:- Wistar rats (450-550 g) individually housed or with one cage mate. 14/10 light/dark cycle.
- In principle, the method can be applied to any whiskered animal, but bleaching darker whiskers could require longer time and could produce less satisfactory results, affecting the quality of the fluorescence.
- Wistar rats (450-550 g) individually housed or with one cage mate. 14/10 light/dark cycle.
- Epigel (Ceva, Italy)
- Hydrogen peroxide (diluted solution 3%)
- Blue bleaching powder (DECO+)
- Distilled water (Marco Viti Farmaceutici)
- Kitchen vinegar
- Domitor (Medetomidine hydrochloride)
- Antisedan (Atipamezole hydrochloride)
Equipment
- Cage for rat (Tecniplast, catalog number: 1291H )
Note: Rat is isolated in cage.
- Animal scale
- Pulse-oximeter
- 1 x scissor (Swiss Scissors, 9 cm) (World Precision Instruments, catalog number: 504613 )
- 1 x tweezer (#7, World Precision Instruments, catalog number: 504504 )
- Tissue and heating pad for body thermoregulation (Panlab s.l.)
- Fluorescent dye
Note: We used and tested hair semi-permanent fluo rinse (‘UV Red’ or ‘UV Green’, Star Gazer).
- Excitation light
Note: We used a custom made lamp with 7 bulbs: ILH-OO01-DEBL-SC211-WIR200, Wavelength 455 nm, Flux @700 mA 1,400 mW, Radiance angle ± 60°; concentrator lens FA11205_Tina-D-OSL FWHM angle ± 6°. The light was custom made to yield uniform illumination from different directions: this improves the homogeneity of the light emitted by the fluorescent dye and then improves the image quality. Each bulb was assembled using a commercial LED coupled with a compound parabolic concentrator (CPC), also called a ‘Winston cone’, in order to collect and project into the arena all the available light.
- Black neoprene (RS Components, catalog number: 733-6753 )
Note: We covered the experimental setup with black neoprene because a black, non-reflective, background helps in increasing the overall contrast of the image.
- Safety goggles (Univet, catalog number: 5X7.03.00.04 )
Note: Absorbance > 4 for Wavelength < 510 nm. Certification 0068/ETI-DPI/070-2009 Rev.2.
- High-speed camera and lens
Note: Camera and lens depend on the experimenter’s choice. We used the camera CamRecord 450 (Optronis) combined with Computar TV lens–50 mm 1:1.3 lens. Each investigator must choose the video device according to frame-rate required by the experiment: for high resolution rat whisker kinematics 1,000 fps is an acceptable frame rate (Fassihi et al., 2014 and 2017). Attention has to be paid to the camera frame rate: the higher the rate, the stronger the light should be. For a frame rate of 1,000 fps, the duration of a single frame is 1 msec, giving the maximum exposure time. We used the entire frame duration, 1 msec, as exposure time. Lower exposure times typically generate better still images but require additional light: a trade-off has to be optimized according to the specific requirements of each individual experimental setup. In case the experimenter wants to reduce the intensity of the stimulus light, or in case a different (e.g., less bright) camera lens is used, some cameras allow setting of the internal gain of the sensor: this improves the sensitivity of the camera sensor at the cost of getting more salt-pepper noise in the resulting images.
- Long-pass filter
Note: Long-pass filter is needed to visualize one or multiple whiskers stained with different dyes. We tested FEL0500 Long-pass filter cut-on wavelength 500 nm (Thorlabs) to visualize multiple fluorescent dyes and red/orange plexiglass to visualize only the red fluorescent dye. The filter choice should fit the lens dimension, while plexiglass, which is sold in sheets, can be cut to suit custom design, then it can be adapted to the lens dimension. As for camera and lens, the filter has to be chosen to match the dye color and suppress the color of the illumination source. This prevents the camera sensor from being bloomed by the source, while receiving the lower-intensity light emitted by the fluorescent dye.
Procedure
- Dye application
Notes:- No sterile techniques are required for this protocol.
- Wear gloves and mask during the entire procedure.
- Prepare in advance a fresh hair discoloration preparation:
- Considering that it has an unpleasant and strong smell, you might consider using a hood.
- Fill at least one PCR tube with bleaching powder and the hydrogen peroxide, close the tube and mix it.
- A viscous preparation must be obtained to avoid it dripping off the whisker.
- Considering that it has an unpleasant and strong smell, you might consider using a hood.
- Prepare in advance cotton pads (or gauzes) soaked in distilled water and vinegar.
- Wear protective goggles before switching the excitation source on.
- Place the rat on the heating pad and set the body temperature to 37 °C.
- Put the Epigel on the rat’s eyes to protect them from light and dryness.
- Apply the discoloration preparation (Figure 1A) on all whiskers using cotton swabs. Since its smell is quite strong, avoid placing it close to the rat’s nose, because breathing it can reverse the effect of Domitor.
- Wait between 30 min and 1 h to bleach whiskers.
- Rinse the whiskers using distilled water with a cotton pad (Figure 1B).
- Dry the whiskers with air-flow or a cotton pad (Figure 1C).
- Place the rat’s head under illumination and tilt it a bit to reach the whisker(s) you are interested in.
- Isolate selected whiskers (Figure 1D)
- Cut a piece of Parafilm to cover the rat’s snout and cheeks (e.g., 6 cm L x 6 cm H).
- Make a small hole to let the whisker pass through. You can use a glass capillary or a tweezer to isolate the whisker, in both cases paying attention not to damage the whisker or the rat’s skin.
- The resulting working surface allows application of the dye(s) only on a selected subset of whiskers. This is very important in order to prevent the unwanted application of the dye.
- Cut a piece of Parafilm to cover the rat’s snout and cheeks (e.g., 6 cm L x 6 cm H).
- Flatten the working surface at the base of the selected whisker(s) to allow the application of the dye on the complete whisker(s) length.
- Apply the fluorescent dye on whisker(s) (Figure 1E). For each whisker:
- Cut a piece of Parafilm of circa 2 cm width and slightly longer than the whisker.
- Fill the syringe with the fluorescent dye and eject it abundantly onto the Parafilm in order to soak the whisker along its entire length. In order to avoid air bubbles, which would locally prevent staining, spread the dye with a cotton swab.
- The whisker now adheres weakly to the Parafilm because soaked in a gel. Any movement can make the whisker slip away. It will be helpful to hold the Paraffin film or even tighten it with aluminum foil.
- Cut a piece of Parafilm of circa 2 cm width and slightly longer than the whisker.
- Wait between 30 min and 1 h from the last application to let the dye diffuse inside the cuticle.
- Remove the excessive dye and rinse the whisker(s) using a cotton pad or a gauze wet with vinegar, which tends to close the cuticle in complete safety for the animal.
Notes:- Clean one whisker at a time. Use a different cotton pad (or gauze) for each whisker in order to not mix the color).
- Since the vinegar smell is quite strong, avoid placing it close to the rat’s nose, because breathing it can reverse the effect of Domitor.
- Clean one whisker at a time. Use a different cotton pad (or gauze) for each whisker in order to not mix the color).
- Visualization test (Figure 1F): wear the protective glasses, turn off all the room lights and turn on the lamp.
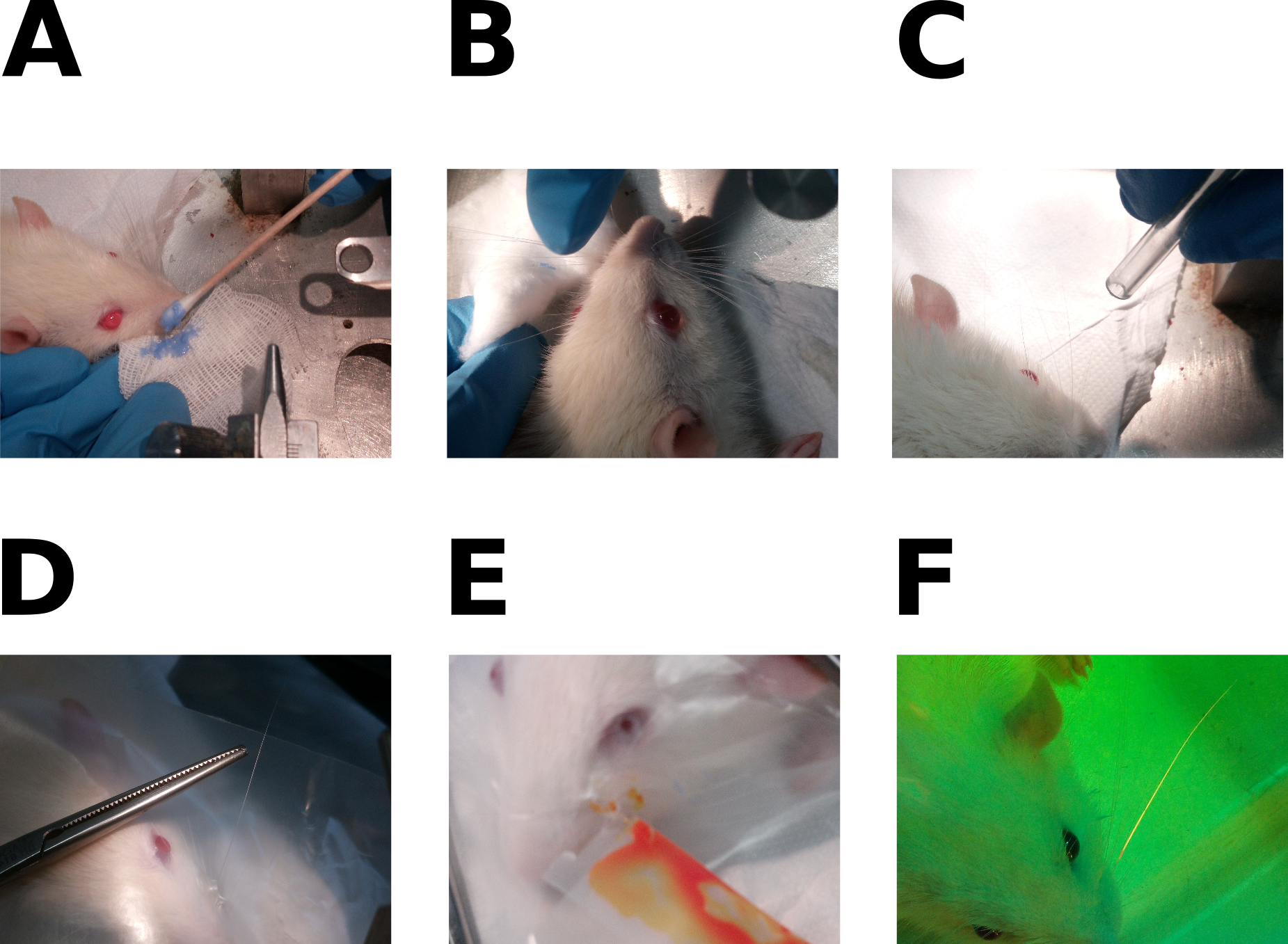
Figure 1. Dye application procedure. A. Whisker bleaching; B. Removal of bleaching factor; C. Whiskers drying in airflow; D. Isolation of a single whisker with paraffin film; E. Application of the dye; F. Visualization test. This figure was adapted using images from Supplementary Figure 1 of Rigosa et al., 2017.
- Set the shutter speed of the high speed camera according to experimenter’s needs.
Note: The higher the shutter speed, the shorter the exposure time, the darker the grabbed image. Choose the highest exposure time possible for brightest images.
- Simulate experimental conditions placing the animal in a box covered with neoprene and installing the lamp at the proper distance upon experimenter’s needs.
Note: Light conditions and reflections strongly depend on the neoprene and lamp installation.
- Visualize the whisker(s) and adjust the camera gain (Figures 2 and 3).
Note: If the background is much darker than the visualized whisker, the gain of the camera sensor can be safely increased without introducing salt and pepper noise to the image. This feature depends on the specific camera model.
- Set the shutter speed of the high speed camera according to experimenter’s needs.
- Follow the guidelines for Post-anesthesia (see Procedure B: ‘Animal sedation’) to recover the rat.
- In case the visualization is not sufficient (e.g., the whisker did not absorb the dye along its entire length), plan another application of the dye. In case the time left before the current sedation ends is not sufficient for immediate re-staining, ask your veterinarian when another sedation for the same animal could be feasible.

Figure 2. High-speed video setup. A cage equipped with a licking sensor is used to trigger and synchronize the high-speed video recording with the licking behavior. The field of view is illuminated using the custom made lamp and the optical path to the camera sensor was filtered using an orange transparent plexiglass.
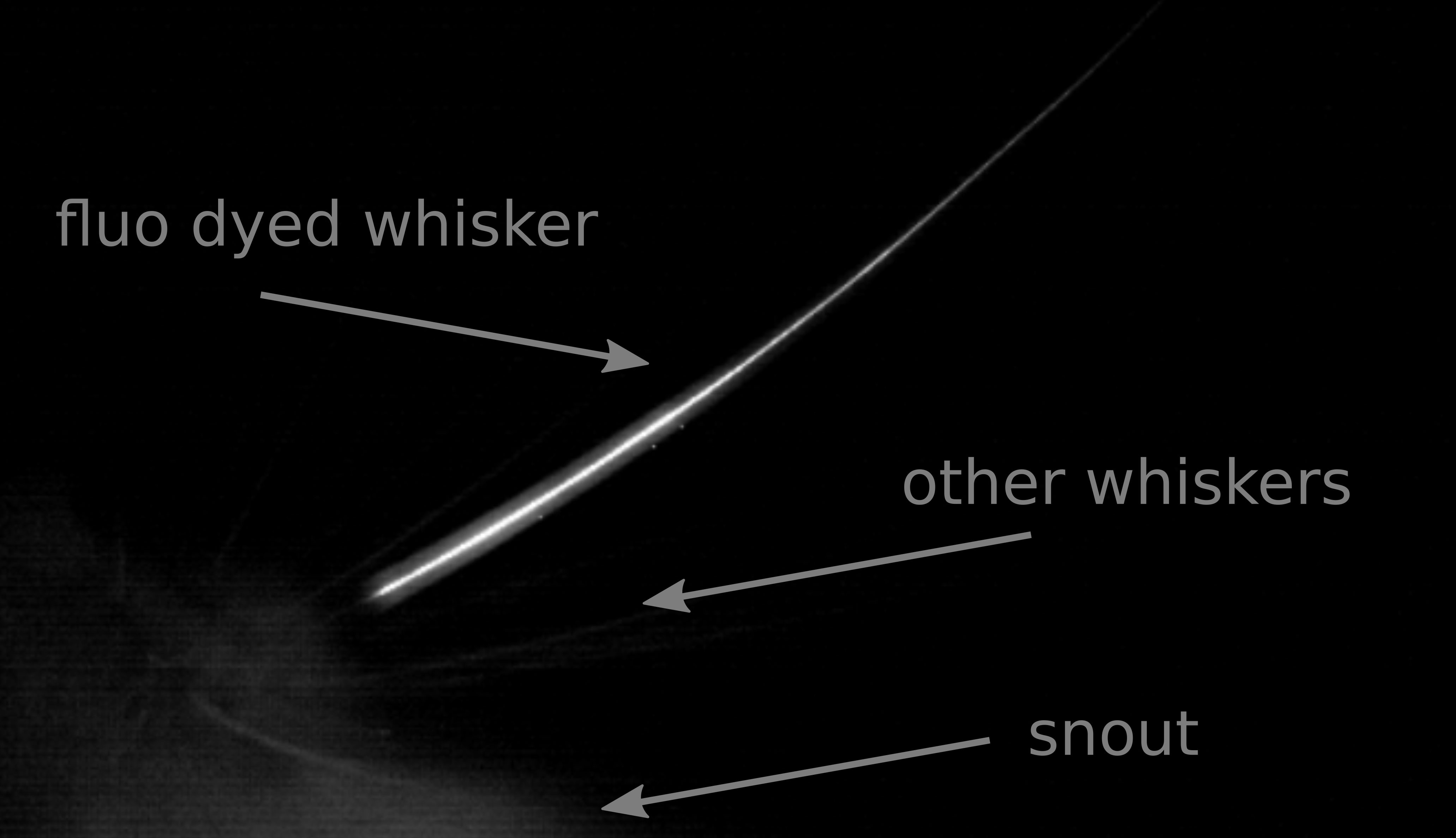
Figure 3. Grabbed frame from high-speed video. A behaving rat is illuminated using 4 LEDs of the lamp and an orange plexiglass plate as an optical filter. The video was shot at 1,000 frames per second. This figure was adapted using images from Supplementary Figure 3 of Rigosa et al., 2017.
- No sterile techniques are required for this protocol.
- Animal sedation
Notes:- This protocol was performed without sterile techniques.
- Inform yourself about the features of Domitor: time of effect, site of injection, which reflexes are suppressed and time of recovery.
- Set ambient temperature to the room temperature (25 °C) and keep it constant.
- Wear gloves and mask during the entire procedure.
- The sedation should last between 2.5 and 3 h.
- Between two sedations the rat needs 24 h of recovery.
- Avoid more than two sedations a week.
- Do not obstruct the animal breathing with anything while it is sedated.
- Do not disturb the animal sedation with strong odors (e.g., by approaching the discoloration preparation or the vinegar).
Pre-anesthesia procedure- Make sure that the rat drank water in the last 5 h.
- Make sure that the rat is housed alone in the cage.
- Bring the rat to a silent and quiet place with constant room temperature.
- Weigh the rat and calculate the dose for the anesthetic; the dose of Domitor (Medetomidine hydrochloride) is 0.5 mg/kg [1 mg/ml] intraperitoneal (IP).
- Prepare the anesthetic in the syringe, ready to be injected.
Anesthesia procedure- From now on, the procedure should be as fast as possible to reduce the risk of stressing the animal.
- Make the IP injection:
- Restrain the animal, expose the abdomen and inject in the lower right quadrant to avoid inner organs.
Note: In alternative, grasp the rat by the tail and raise its belly, with its posterior legs lifted, inner organs will move towards the head, reducing the risk of missing the target of the shot.
- Don’t keep the animal in the restrained position for a long time; it is unnatural and stressful.
- Inject the anesthetic IP in a single shot.
- Restrain the animal, expose the abdomen and inject in the lower right quadrant to avoid inner organs.
- Place the rat in the cage
- Use a heating pad or cover the rat body with a tissue or aluminum foil to diminish heat dissipation.
- Place the cage in a dark room.
- Use a heating pad or cover the rat body with a tissue or aluminum foil to diminish heat dissipation.
- After 10 min test the reflexes (tail, hind paw, corneal).
- If the animal is sedated, apply the dye.
- During sedation, constantly monitor the heart beating and the breathing rate.
Post-anesthesia procedure- Put the rat back in the cage.
- Prepare the dose of the Antisedan (Atipamezole hydrochloride), standard dose for IP injection is 0.2 ml.
- Make the IP injection.
- Cover the rat with a tissue to keep its body temperature at 37 °C until it wakes up.
- This protocol was performed without sterile techniques.
Notes
The whisker staining (protocol ‘Dye application’) is executed on the sedated animal (protocol ‘Animal sedation’). The sedation should last between 2.5/3 h and between two sedation there should be a resting period of at least 24 h, and in any case no more than twice a week.
The dye is subject to photo-bleaching. Though in Rigosa et al. (2017) a time constant of about 14 h was reported (i.e., ca. 25,000 trials of 2 sec each), this result depends on the light density and the amount of dye that was absorbed by the whisker sample. If necessary, the investigator can re-stain the same whisker, as this would not alter its mechanical properties. If staining multiple whiskers, the investigator can obtain the same result by staining one whisker at a time across different sessions.
Acknowledgments
The authors declare no competing financial interests. We acknowledge the financial support of the Human Frontier Science Program (http://www.Hfsp.org; project RG0015/2013), the European Research Council Advanced grants CONCEPT (http://erc.europa.eu; project 294498) and MicroMotility (project 340685), and Italian MIUR grant HANDBOT (http://hubmiur.pubblica.istruzione.it/web/ricerca/home; project GA 280778). GN gratefully acknowledges support by SISSA through the excellence program NOFYSAS 2012. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Fassihi, A., Akrami, A., Esmaeili, V. and Diamond, M. E. (2014). Tactile perception and working memory in rats and humans. PNAS 111: 2331-2336.
- Fassihi, A., Akrami, A., Pulecchi, F., Schönfelder, V. and Diamond, M. E. (2017). Transformation of perception from sensory to motor cortex. Curr Biol 27: 1585-1596.
- Rigosa, J., Lucantonio, A., Noselli, G., Fassihi, A., Zorzin, E., Manzino, F., Pulecchi, F. and Diamond, M. E. (2017). Dye-enhanced visualization of rat whiskers for behavioral studies. Elife 6.
Article Information
Copyright
Rigosa et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Rigosa, J., Lucantonio, A., Noselli, G., Fassihi, A., Zorzin, E., Manzino, F., Pulecchi, F. and Diamond, M. E. (2018). A Fluorescent Dye Method Suitable for Visualization of One or More Rat Whiskers. Bio-protocol 8(5): e2749. DOI: 10.21769/BioProtoc.2749.
- Rigosa, J., Lucantonio, A., Noselli, G., Fassihi, A., Zorzin, E., Manzino, F., Pulecchi, F. and Diamond, M. E. (2017). Dye-enhanced visualization of rat whiskers for behavioral studies. Elife 6.
Category
Neuroscience > Behavioral neuroscience > Learning and memory
Neuroscience > Sensory and motor systems > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









