- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Assaying Mechanonociceptive Behavior in Drosophila Larvae
Published: Vol 8, Iss 4, Feb 20, 2018 DOI: 10.21769/BioProtoc.2736 Views: 10081
Reviewed by: Jay Z ParrishAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assaying Thermo-nociceptive Behavior in Drosophila Larvae
Meike Petersen [...] Peter Soba
Feb 20, 2018 8686 Views

Accessing Olfactory Habituation in Drosophila melanogaster with a T-maze Paradigm
Ourania Semelidou [...] Efthimios M.C. Skoulakis
Jun 5, 2019 7851 Views

Rearing and Shipping of Uranotaenia lowii, a Frog-Biting Mosquito
Richa Singh [...] Ximena E. Bernal
Jun 5, 2024 1425 Views
Abstract
Drosophila melanogaster larvae have been extensively used as a model to study the molecular and cellular basis of nociception. The larval nociceptors, class IV dendritic arborization (C4da) neurons, line the body wall of the animal and respond to various stimuli including noxious heat and touch. Activation of C4da neurons results in a stereotyped escape behavior, characterized by a 360° rolling response along the body axis followed by locomotion speedup. The genetic accessibility of Drosophila has allowed the identification of mechanosensory channels and circuit elements required for nociceptive responses, making it a useful and straightforward readout to understand the cellular and molecular basis of nociceptive function and behavior. We have optimized the protocol to assay mechanonociceptive behavior in Drosophila larvae.
Keywords: NociceptionBackground
Nociception, the innate ability to detect and avoid noxious stimuli, is highly conserved across the animal kingdom. Drosophila melanogaster larvae are capable to detect and avoid a variety of noxious stimuli including noxious touch, heat and light (Tracey et al., 2003; Hwang et al., 2007; Xiang et al., 2010). Mechanical stimulation above a certain threshold (> 30 mN) elicits a stereotyped rolling escape response at all larval stages (Almeida-Carvalho et al., 2017), which is thought to have evolved to avoid ovipositor injection by parasitic wasps such as L. boulardi (Hwang et al., 2007). This escape response is mediated by activation of nociceptive C4da neurons, which possess sensory dendrites covering the entire body wall allowing the animal to detect noxious cues. C4da neurons express several mechanosensory channels belonging to the DEG/ENaC family (pickpocket [ppk], ppk26/balboa) (Zhong et al., 2010; Gorczyca et al., 2014; Guo et al., 2014; Mauthner et al., 2014), a mechanosensitive TrpA1 isoform (Zhong et al., 2012), piezo (Kim et al., 2012) and the Trp channel painless (Tracey et al., 2003), all of which are required for normal mechanonociceptive responses.
The escape response can be assayed by using a von Frey filament exerting a force between 30-120 mN, which activates mechanosensory channels in C4da neurons (Hwang et al., 2007; Kim et al., 2012). Recent work has also shed light on circuit mechanisms required for mechanonociceptive responses. Mechanically induced escape responses require co-activation of class II da (C2da) and class III da (C3da) sensory neurons, as silencing of either subset impaired rolling behavior (Hu et al., 2017). Moreover, this sensory integration is specific for mechanonociception and in addition requires neuropeptide-mediated feedback. We provide a detailed protocol from our recent work (Hu et al., 2017), which employed a mechanonociception assay based on previously described methods (Hwang et al., 2007; Caldwell and Tracey, 2010; Zhong et al., 2010). We typically use a mechanical force of 45-50 mN, which elicits weak responses after the first stimulus, but enhanced responses after a second subsequent stimulus. This approach allows assaying changes in sensitivity and sensitization of mechanonociceptive responses, which can be coupled with genetic approaches to identify molecular and network components required for normal escape behavior.
Materials and Reagents
- Drosophila vials (wide, K-Resin) (Dutscher, catalog number: 789002 )
- Flugs® fly plugs, plastic vials (wide) (Dutscher, catalog number: 789035 )
- Omniflex monofilament fishing line Shakespeare (6 lb test, diameter 0.23 mm) (Zebco, Tulsa, USA)
- Petri dishes (Ø 10 cm) (SARSTEDT, catalog number: 82.1473 )
- Fly stocks
Chromsome, Bloomginton stock center No.:
w1118 (X, BL 6326)
w*; ppk-Gal4 (X, 3rd, BL 32079)
w*;UAS-TNTE (X, 3rd, BL 28997)
w*; TrpA11 (X, 2nd , BL 36342) - Agar Kobe I (Carl Roth, catalog number: 5210.4 )
- Agar plates (see Recipes)
- Fly food (see Recipes)
- Agar (strings) (Gewürzmühle Brecht, Eggenstein, catalog number: 00262 )
- Corn flour (Davert, Newstartcenter, catalog number: 17080 )
- Soy flour (Davert, Newstartcenter, catalog number: 46985 )
- Brewer’s yeast (ground) (Gewürzmühle Brecht, Eggenstein, catalog number: 03462 )
- Malt syrup (MeisterMarken–Ulmer Spatz, Bingen am Rhein, catalog number: 728985 )
- Treacle (molasses) (Grafschafter Krautfabrik, Meckenheim, catalog number: 01939 )
- Nipagin (Methyl 4-hydroxybenzoate) (Sigma-Aldrich, catalog number: 54752-1KG-F )
- Propionic acid (Carl Roth , catalog number: 6026.3 )
- Agar (strings) (Gewürzmühle Brecht, Eggenstein, catalog number: 00262 )
Equipment
- Brush (Size 1, Boesner, model: Da Vinci Nova Serie 1570 , catalog number: D15701)
- Forceps (Dumont, #3) (Fine Science Tools, catalog number: 11231-30 )
- Light source (white light) LED Schott KL 1500 LCD (Pulch und Lorenz, catalog number: 150.200)
Manufacturer: SCHOTT, model: KL 1500 LCD . - SZX7 stereo microscope (Olympus, model: SZX7 )
Software
- Origin Pro 9.0 (OriginLab, Northampton, USA) or similar for statistical analysis
Procedure
- All fly stocks were maintained at 25 °C and 70% humidity with a 12 h dark/light cycle on standard fly food. All experiments were performed using 3rd instar larvae at 96 h (hours) after egg laying (AEL). In order to ensure that all larvae were about the same age, the egg laying was restricted to 4-6 h. Experimental crosses were raised on standard fly food at 25 °C with 70% humidity and a 12 h light/12 h dark cycle.
- Prepare your genetic crosses 2 days before staging with approx. 20-30 virgins and 10-15 males each (more if you are using weak genotypes). After 2 days, transfer flies to a fresh food vial for timed egg-laying for 4-6 h at 25 °C (Figure 1A). Transfer the adult flies to a fresh vial (for another round of staging on the same or next day). The original vial is maintained at 25 °C until 96 ± 3 h AEL. All larvae should be in the third instar (L3) foraging stage and not yet leaving the food (Figure 1B).
Note: Precise staging is important, as nociceptive responses of larvae are reduced after 120 h AEL, likely due to the transition to the wandering stage and preparation for pupariation. The density of larvae in the food will also affect staging: too few animals cannot efficiently process the food, while having too many larvae will result in competition for food, both of which is affecting developmental progression and will broaden the developmental stage of the larval population. In case mutant animals with delayed development are used staging has to be adjusted accordingly (staging either by animal size or molting counting mouth hook teeth).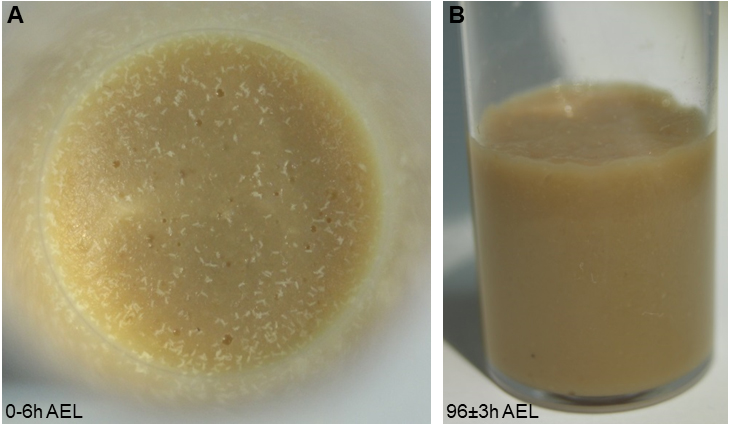
Figure 1. Correct staging and larval density. A. A photograph showing appropriate embryo numbers for the used vial size (approx. 100-150 embryos laid within 4-6 h). B. At 96 h AEL, larvae should have processed the food well and still be in the foraging stage. - Tool preparation
- Cut the omniflex monofilament fishing line (Shakespeare, 6 lb test, diameter 0.009 inch [0.23 mm]) to a length of 18 mm. 10 mm is attached to a toothpick such that 8 mm of the fiber protruded from the end of the toothpick (Figure 2A).
- Calibrate the force of the fiber by using it to depress a balance until the fishing line is seen to bend. Record the force (in grams) and convert to milli-Newtons (mN) by multiplying the measured grams by a factor of 9.81. Typically, this length of filament results in a force of 45-50 mN (Figures 2B-2D). Varying the filament length will change the mechanical force (the longer, the less the force), which might be desirable if different forces should be tested. The probability of nociceptive responses of larvae increases with the applied force from 30 to 120 mN (Kim et al., 2012; Almeida-Carvalho et al., 2017).
- Examine the filament under a stereoscope and make sure that no sharp edges remain, which might potentially injure the animal. Puncturing the body wall will result in altered behavioral responses.

Figure 2. Tool preparation and calibration. A. 8 mm filament attached to toothpick prepared according to instructions above. B-D. Calibrating the filament: the filament should exert a force of 45-50 mN (4.59-5.10 g). B. Repeated depression of the filament should result in comparable forces (here 46 ± 5 mN). B’. Replicable forces are achieved by correct pressure and bending of the filament at the right angle as shown (approx. 45-60°). C’. Too little pressure and/or wrong angle of deflection result in lower forces. C’. Too much pressure and/or wrong angle of deflection result in too high forces.
- Cut the omniflex monofilament fishing line (Shakespeare, 6 lb test, diameter 0.009 inch [0.23 mm]) to a length of 18 mm. 10 mm is attached to a toothpick such that 8 mm of the fiber protruded from the end of the toothpick (Figure 2A).
- Distribute 2 ml of dH2O on a 2% agar plate to create a thin water film, which enables the animals to crawl freely and perform their rolling behavior. Without water, the larvae are not fully mobile and do not display consistent behavioral responses.
- Prepare 10-20 staged larvae by washing in dH2O to remove any residual food and place them gently on the agar plate using a brush (Figures 3A-3D).
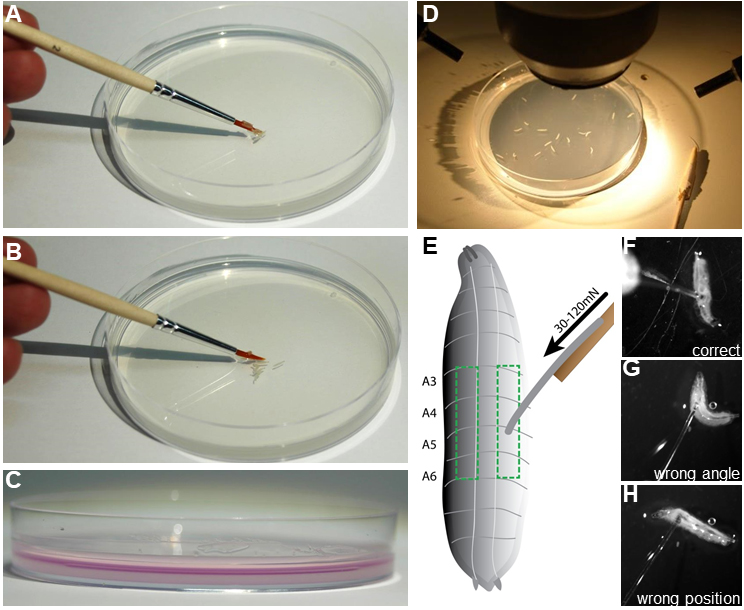
Figure 3. Preparation of mechanonociception assay. A and B. The assay is prepared by gently placing 10-20 staged 3rd instar larvae on a 10 cm 2% agar plate using a brush. C. A thin water film of 2 ml (colored magenta for illustration) is necessary for consistent behavioral responses. D. Agar plate with test animals is placed under a stereoscope with a light source. E. Illustration of a 3rd instar larvae. Boxed region indicates the dorsolateral region of abdominal segments A3-A6, which should be targeted with the filament calibrated to the chosen force (30-120 mN). F-H. Examples of correct and incorrect placement of the filament on the larva are shown. F. Correct placement on dorsolateral A3 region. G. Wrong placement with animal moving away from filament resulting in a bad angle for the stimulus. H. Filament placement at an anterior segment which generally does not elicit rolling behavior. - Deliver the mechanical stimulus by rapidly depressing the larva with the filament on the dorsal side (abdominal segments four, five, or six) for approximately 1 sec. The quick release allows the larvae to perform escape behavior. Reapply the stimulus to the same larva after a pause of 2-3 sec.
- Score the response immediately on a scoring sheet according to Hwang et al. (2007): no response, stop, stop and turn (non-nociceptive responses), or rolling (nociceptive response); in addition, we introduced bending to score for an incomplete nociceptive response (C-shaped simultaneous convulsive head and tail movements) that did not result in rolling (response classification: 1 = no response, 2 = stop, 3 = stop and turn, 4 = bending, 5 = rolling). A positive rolling response is scored if at least one 360° rotation along the body axis occurred in response to the mechanical stimulus (Figures 4A-4D; Videos 1 and 2).
Note: To develop and entrain precision in applying the correct force to the animal the experimenter should practice the correct motion on a balance. Hold the toothpick with the filament and depress the filament at a 45-60° angle until it bends, which should result in a consistent force every time. Practicing with control animals will further ensure consistent results. 50-70% of the animals should respond with rolling escape behavior after the 2nd stimulus.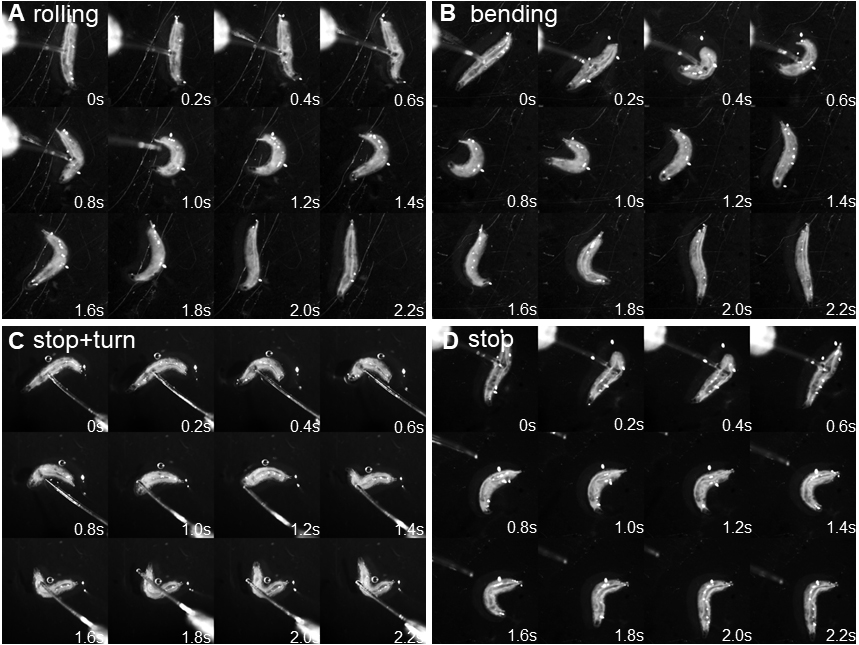
Figure 4. Behavioral responses to mechanonociceptive stimulation. A. Montage of nociceptive rolling response after mechanical stimulation. Note that the larva performs a full 360° roll along the body axis as visible following the main trachea. B. Montage of a partial nociceptive response (‘bending’) resulting in C-shaped body bending but no full 360° roll. C. Montage of stop and turn response (stop and at least 45° change in direction). D. Montage of stop response (no major change in direction of movement).Video 1. Behavioral responses to mechanical stimulation using a 50 mN von Frey filament (all 5 categories)Video 2. Side by side comparison rolling vs. bending
Data analysis
- Statistical differences in mechanonociceptive responses can be calculated using a χ2-test, which allows comparing categorical data between 2 genotypes (e.g., control vs. TrpA11). Distinguishing only nociceptive and non-nociceptive behaviors allows the use of a 2 x 2 contingency table providing the highest statistical power using a χ2-test with 1 degree of freedom. χ2 can be determined according to the following formula:
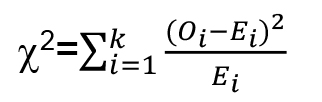
O: observed values, E: expected value.
The χ2-test requires the actual number of animals, as it cannot compute ratios, percentages or frequencies. Most statistics programs (Origin Pro, SigmaPlot, Prism, SAS, Statistica, R) can be used to calculate χ2 and compute statistical significances.
Note: In cases where more than two genotypes should be compared, appropriate correction for multiple comparisons of statistical significances should be performed, e.g., a Bonferroni correction factor (significant if p < a/n, typically with a = 0.05 and n being the number of compared hypotheses). - At least 60 animals per genotype should be tested to ensure that an effect of 20% or greater is resulting in statistical significance (P < 0.05) with sufficient power of the χ2-test (> 0.9).
Note: All behavioral experiments should be blinded and randomized to avoid unwanted bias. The genotypes for the mechanonociception assay should be coded (e.g., numbers or letters), with the experimenter being unaware of the genotypes being tested.
Expected results
- The functionality of the mechanonociception assay can be assessed by inactivation of C4da neurons, either by genetic silencing using Tetanus toxin light chain (UAS-TnT), the inward rectifying potassium channel Kir2.1, or genetic mutants of mechano-sensitive channels (e.g., TrpA11) (Hwang et al., 2007; Zhong et al., 2012; Hu et al., 2017).
- Performing the mechanonociception assay with TrpA11 mutant larvae showed a decrease in nociceptive responses compared to control w1118 larvae (Video 3). After the first stimulation already 76% of control larvae displayed nociceptive responses (bending + rolling behavior) (Figure 5A). In contrast, none of TrpA11 larvae showed nociceptive responses to the mechanical force. Treating the larvae a second time resulted in 85% nociceptive response (50% rolling), whereas only 10% of TrpA11 animals reacted with nociceptive bending but no rolling (Figure 5B). Video 3. Exemplary behavioral response of TrpA11 animals to mechanical stimulation using a 50 mN von Frey filament
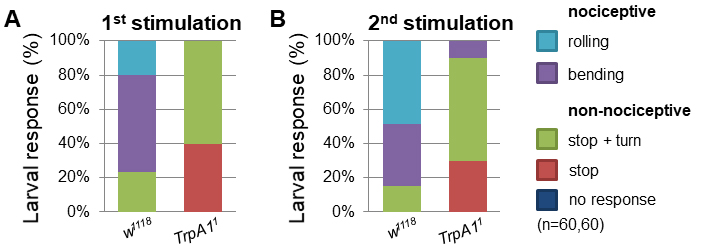
Figure 5. TrpA11 mutant larvae exhibited defects in mechanonociceptive behavior. Response of 3rd instar larvae to a mechanonociceptive stimulus (50 mN). The behavioral response was categorized into nociceptive (rolling, bending) and non-nociceptive responses (stop and turn, stop, no response). Behavioral differences between nociceptive and non-nociceptive behavior of 3rd instar control and TrpA11 larvae after the 2nd stimulation were compared using a χ2-test. P < 0.0001 (χ2-test, 1 degree of freedom, n = 60/genotype). - Alternatively, cell type specific genetic manipulation or silencing can be employed to explore circuit function by employing expression of the tetanus toxin light chain (UAS-TnT). C4da specific expression of TnT (ppk-Gal4/UAS-TnT) resulted in mechanonociceptive defects, displayed by a decrease in nociceptive behavior and an increase in non-nociceptive responses compared to control animals (Figure 6).
- These results confirmed the experimental design and C4da neuron function in mechanonociception. This approach should allow the identification of genes required for mechanonociception in C4da or downstream neurons of the nociceptive network.
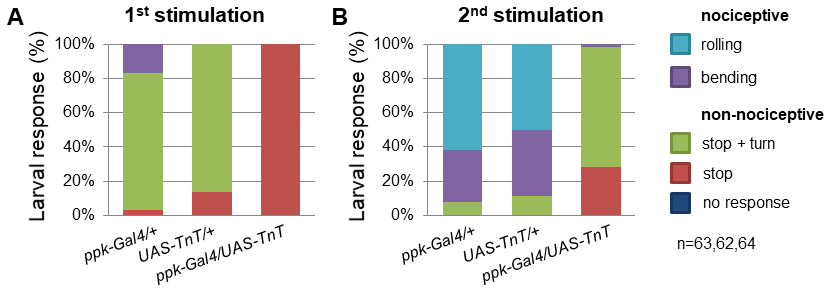
Figure 6. C4da neuron silencing with TnT impairs mechanonociceptive behavior. Percentage of 3rd instar larvae showing categorized responses after mechanonociceptive stimulation (50 mN). Behavioral responses of animals with C4da neuron-specific overexpression of TnT (ppk-Gal4/UAS-tnt) and controls were compared. Responses were categorized into nociceptive (rolling, bending) and non-nociceptive responses (stop and turn, stop, no response). Blocking synaptic transmission in C4da neurons by TnT expression resulted in strong defects in mechanonociceptive responses after the 2nd stimulation. P < 0.0001(***) (χ2-test, 1 degree of freedom, n = 63, 62, 64).
Notes
Under optimal conditions (proper staging, same filament, etc.) results are highly reproducible between experiments performed on different days and by different trained experimenters. From our experience, tight staging, density-controlled vials and gentle handling of the animals are critical for reproducible results. Behavioral response rates are highly dependent on applying the stimulus consistently and appropriately. Due to the manual procedure it takes practice to be able to consistently stimulate larvae. We recommend practicing with a balance first to ensure that the applied force is constant. Next, practicing with wildtype and mutant larvae displaying impaired mechanonociception (e.g., TrpA11 mutant larvae) will ensure consistent and reproducible behavioral responses.
Recipes
- Agar plates
Note: For mechanonociception assays, 2% agar plates were used.
Dissolve Kobe agar I in dH2O and fill Petri dishes (Ø 10 cm) with a defined volume of 12 ml - Fly food
Use the following ingredients for 1 L of standard fly food:
Ingredients for 1 L of standard fly food: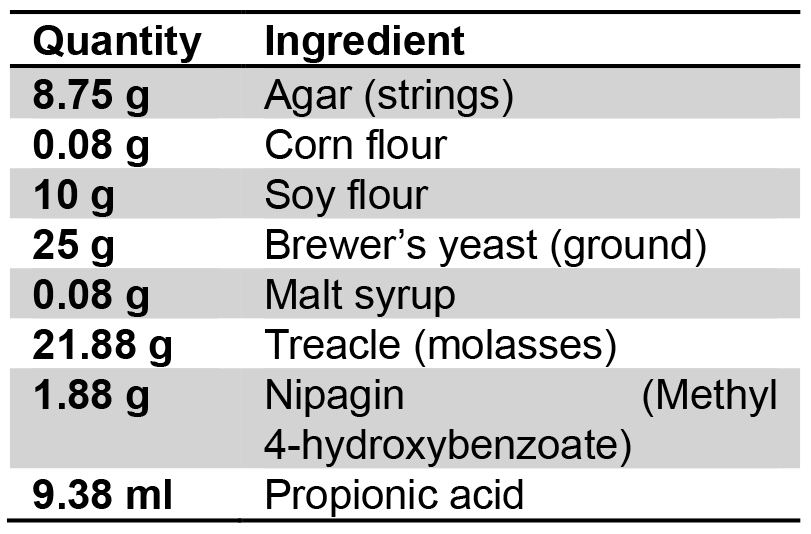
Dissolved in 1 L dH2O
Acknowledgments
This work was supported by the Landesforschungsförderung LFF-FV27 (to P.S.) and the Deutsche Forschungsgemeinschaft priority program SPP1926 (project SO1337/2-1 to P.S.). The authors would like to acknowledge the previous work and effort of several groups developing and applying mechanonociceptive assays in Drosophila, in particular the labs of D. Tracey, A. Patapoutian, Y.N. Jan and Z. Wang. The authors declare that no conflicts of interest or competing interests exist.
References
- Almeida-Carvalho, M. J., Berh, D., Braun, A., Chen, Y. C., Eichler, K., Eschbach, C., Fritsch, P. M. J., Gerber, B., Hoyer, N., Jiang, X., Kleber, J., Klambt, C., Konig, C., Louis, M., Michels, B., Miroschnikow, A., Mirth, C., Miura, D., Niewalda, T., Otto, N., Paisios, E., Pankratz, M. J., Petersen, M., Ramsperger, N., Randel, N., Risse, B., Saumweber, T., Schlegel, P., Schleyer, M., Soba, P., Sprecher, S. G., Tanimura, T., Thum, A. S., Toshima, N., Truman, J. W., Yarali, A. and Zlatic, M. (2017). The Ol1mpiad: concordance of behavioural faculties of stage 1 and stage 3 Drosophila larvae. J Exp Biol 220(Pt 13): 2452-2475.
- Caldwell, J. C. and Tracey, W. D. (2010). Alternatives to mammalian pain models 2: Using Drosophila to identify novel genes involved in nociception. Methods Mol Biol 617: 19-29.
- Gorczyca, D. A., Younger, S., Meltzer, S., Kim, S. E., Cheng, L., Song, W., Lee, H. Y., Jan, L. Y. and Jan, Y. N. (2014). Identification of ppk26, a DEG/ENaC channel functioning with Ppk1 in a mutually dependent manner to guide locomotion behavior in Drosophila. Cell Rep 9(4): 1446-1458.
- Guo, Y., Wang, Y., Wang, Q. and Wang, Z. (2014). The role of PPK26 in Drosophila larval mechanical nociception. Cell Rep 9(4): 1183-1190.
- Hu, C., Petersen, M., Hoyer, N., Spitzweck, B., Tenedini, F., Wang, D., Gruschka, A., Burchardt, L. S., Szpotowicz, E., Schweizer, M., Guntur, A. R., Yang, C. H. and Soba, P. (2017). Sensory integration and neuromodulatory feedback facilitate Drosophila mechanonociceptive behavior. Nat Neurosci 20(8): 1085-1095.
- Hwang, R. Y., Zhong, L., Xu, Y., Johnson, T., Zhang, F., Deisseroth, K. and Tracey, W. D. (2007). Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol 17(24): 2105-2116.
- Kim, S. E., Coste, B., Chadha, A., Cook, B. and Patapoutian, A. (2012). The role of Drosophila Piezo in mechanical nociception. Nature 483(7388): 209-212.
- Mauthner, S. E., Hwang, R. Y., Lewis, A. H., Xiao, Q., Tsubouchi, A., Wang, Y., Honjo, K., Skene, J. H., Grandl, J. and Tracey, W. D., Jr. (2014). Balboa binds to pickpocket in vivo and is required for mechanical nociception in Drosophila larvae. Curr Biol 24(24): 2920-2925.
- Tracey, W. D., Jr., Wilson, R. I., Laurent, G. and Benzer, S. (2003). Painless, a Drosophila gene essential for nociception. Cell 113(2): 261-273.
- Xiang, Y., Yuan, Q., Vogt, N., Looger, L. L., Jan, L. Y. and Jan, Y. N. (2010). Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468(7326): 921-926.
- Zhong, L., Bellemer, A., Yan, H., Ken, H., Jessica, R., Hwang, R. Y., Pitt, G. S. and Tracey, W. D. (2012). Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep 1(1): 43-55.
- Zhong, L., Hwang, R. Y. and Tracey, W. D. (2010). Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol 20(5): 429-434.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Hoyer, N., Petersen, M., Tenedini, F. M. and Soba, P. (2018). Assaying Mechanonociceptive Behavior in Drosophila Larvae. Bio-protocol 8(4): e2736. DOI: 10.21769/BioProtoc.2736.
Category
Neuroscience > Behavioral neuroscience > Sensorimotor response
Neuroscience > Sensory and motor systems > Animal model
Molecular Biology > DNA > Mutagenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











