- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Medaka-microinjection with an Upright Microscope
Published: Vol 8, Iss 3, Feb 5, 2018 DOI: 10.21769/BioProtoc.2716 Views: 8703
Reviewed by: David CisnerosAlberto RissoneMichelle Goody

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

GeneWeld: Efficient Targeted Integration Directed by Short Homology in Zebrafish
Jordan M. Welker [...] Maura McGrail
Jul 20, 2021 11708 Views

Fluorescent PCR–based Screening Methods for Precise Knock-in of Small DNA Fragments and Point Mutations in Zebrafish
Blake Carrington and Raman Sood
Aug 5, 2023 2232 Views

Creating Loss-of-Function Mutants of Neurospora crassa Using a Novel CRISPR/Cas9 System
Stefanie Grüttner
Jan 5, 2026 199 Views
Abstract
We described a simple method for microinjecting DNA/RNA/Protein solutions into medaka eggs under an upright microscope. Medaka is an excellent vertebrate model for reverse genetics, because of its daily spawning, short generation time, and egg transparency. These features enable us to efficiently perform functional genomic analyses of transgenic or genome edited fish. This protocol contains the initial steps necessary to create various types of genetically modified fish.
Keywords: FishBackground
Medaka is a small freshwater teleost species and has desirable features for use as a vertebrate model, including daily spawning, complete genome sequence, and availability of a number of useful strains. Moreover, the transparency of its eggs enables precise introduction of DNA/RNA/Protein solutions into the cytoplasm of the eggs by microinjection. Microinjection is important for the functional genomic analysis; for example, DNA microinjection is an indispensable technique for generating transgenic fish, and helps us to understand the functions of introduced genes in vivo (Ozato et al., 1986). Furthermore, microinjection of CRISPR/Cas system is able to achieve targeted genome editings such as knockout and knock-in approaches (Ansai and Kinoshita, 2014; Murakami et al., 2017a).
Both of a stereoscopic microscope and an upright microscope can be used for microinjection into fish embryos. Although microinjection with an upright microscope requires attachment of some specific instruments including a micromanipulator and an injection needle holder to the microscope, this method may achieve more precise introduction of solutions into the cytoplasm than that with a stereoscopic microscope due to its high resolution property. In this paper, we described the outline of a microinjection method with an upright microscope. This method can be used together with other protocols, such as those for the generation of gene knockout or gene knock-in lines (Ansai and Kinoshita, 2014; Murakami et al., 2017b).
Materials and Reagents
- Spatula (SANSYO, catalog number: 93-4159 or equivalents)
- Cutter (SANSYO, catalog number: 97-0417 or equivalents)
- Glass capillary (NARISHIGE, catalog number: GD-1 )
- Egg holder plate (Figure 1)
- Silicone sealant; repair materials made of silicone (Konishi, catalog number: 04890 or equivalents)
- Micro-loading tip (Eppendorf, catalog number: 5242956003 )
- Disposable syringe (TERUMO, catalog number: 4987350396457 or equivalents)
- Disposable glass pipette (SANSYO, catalog number: 73-0102 or equivalents)
- Needle (Hamilton, catalog number: KF731 )
- Plastic dish (35 mm in diameter) (Corning, Falcon®, catalog number: 351008 )
- Male fish
- Female fish
- Mineral oil (NACALAI TESQUE, catalog number: 23306-84 )
- Methylene blue (NACALAI TESQUE, catalog number: 37125-95 )
- Phenol red (NACALAI TESQUE, catalog number: 26807-21 )
- Sodium chloride (NaCl)
- Potassium chloride (KCl)
- Calcium chloride dihydrate (CaCl2·2H2O)
- Magnesium sulfate heptahydrate (MgSO4·7H2O)
- 17 mM methylene blue (see Recipes)
- 100x embryo culture medium (see Recipes)
- Embryo culture medium (see Recipes)
Equipment
- Forceps (DUMONT, model: 91-3869 or equivalents)
- Micropipette puller (NARISHIGE, model: PC-100 )
- Microinjection system (NARISHIGE, models: M-152 and IM-6 )
- Stereoscopic microscope (Leica Microsystems, model: WILD MZ8 )
- Upright microscope modified to attach micro-manipulator system (Nikon, model: XF-PH-21 )
Procedure
- Acclimatization of fish for spawning
Prepare 10-15 tanks with one pair of male and female adult fish, and acclimate each fish to a new environment for a week.
Note: Fish are kept under a 14/10-h light/dark cycle at 26 °C to promote spawning. - Preparation of an egg holder
- Prepare a commonly used slide glass (Figure 1A).
- Adhere glass chips on the slide glass to make a channel for setting a silicone sealant (Figure 1B).
- Fill the channel (enclosed by the red line in Figure 1C) of the egg holder plate with the silicone sealant, and flatten its surface with a spatula.
Note: It will take overnight to completely solidify the sealant. - Cut out the hardened sealant in parallel with a cutter, and make a channel (enclosed by the blue line in Figure 1D) of about 1.0 mm in width and about 2.0 mm in depth for fitting eggs.
Note: Unlike an agarose plate, the silicone plate can be used permanently.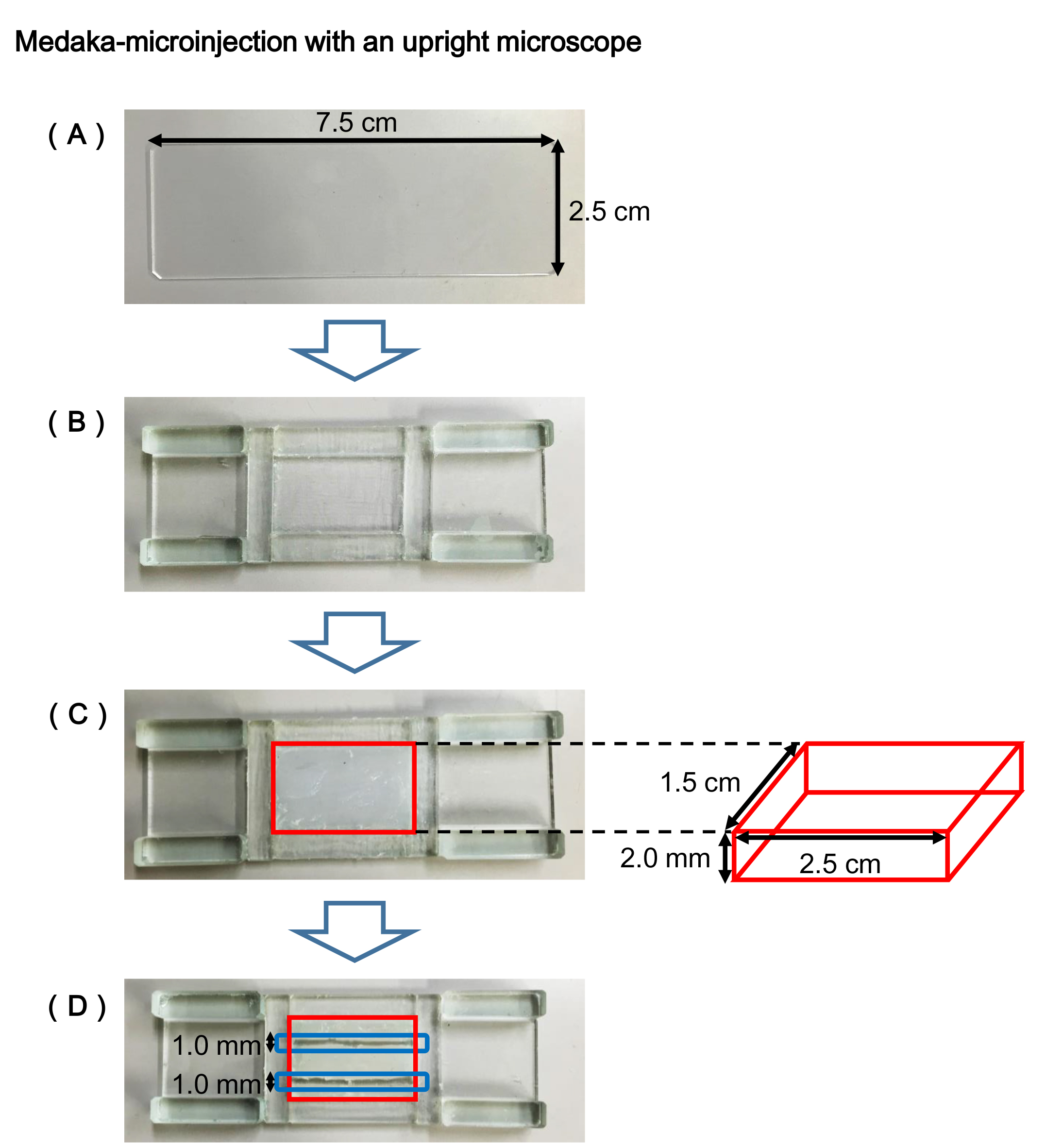
Figure 1. Egg holder plate
- Prepare a commonly used slide glass (Figure 1A).
- Preparation of microinjection in the evening before microinjection
- Pull the glass capillary by the micropipette puller to make several microinjection needles (according to the manufacturer’s instruction) (Kinoshita et al., 2009).
- Set the partition in the fish tank to separate a female from a male preventing from spawning.
- Pull the glass capillary by the micropipette puller to make several microinjection needles (according to the manufacturer’s instruction) (Kinoshita et al., 2009).
- Microinjection procedure in the morning on the day of experiment
- Remove the partition in the fish tank to make the pairs begin spawning.
Note: It will take a few minutes to finish spawning. - Transfer the fertilized eggs to a plastic dish with a disposable glass pipette, and remove the long attaching filaments on the surface of the chorion using forceps under the stereoscopic microscope (Video 1).Video 1. The preparation of fertilized eggs for microinjection (Steps 3a-3b, 4a-4b)
- Load the solution of DNA/RNA or protein in the glass capillary needle using the micro-loading tip.
- Fill the glass capillary needle with mineral oils completely to the end using a disposable syringe fitted with the Hamilton needle.
- Apply pressure to the injector by turning the handle of the injector, and fill completely a line of tubing between injector and injection needle holder with mineral oils, to remove air in the line.
Note: This work helps to prevent the end of the microinjection needle from clogging with air bubbles. - Fix the glass capillary needle in the needle holder for injection, and attach the holder to the micromanipulator (Video 2).Video 2. The loading of the solution in the glass capillary needle, and the attachment of the needle to the micro-manipulator (Steps 4c-4f)
- Transfer the egg to the egg holder with a glass pipette, and place it on the stereoscopic microscope stage.
- Rotate the eggs with forceps to direct its cytoplasm toward the injection needle and fix the egg in the channel of the egg holder on the stereoscopic microscope stage (magnification 20x).
Note: Although it may be difficult to find the cytoplasm just after fertilization, it can become easy to find it gradually, because it progressively accumulates toward the animal pole in the egg; it is easy to find the cytoplasm by observing the opposite side of the droplets that accumulate toward the vegetal pole. - Place the holder on the upright microscope stage and place the cytoplasm at the center of the view by adjusting the stage of microscope (magnification 40x).
Note: If the cytoplasm is not properly oriented toward the injection needle, it is necessary to adjust its position again under the stereoscopic microscope. - Open the end of the needle by touching the end gently to the surface of the egg holder under the microscope.
- Bring the needle close to the cytoplasm by operating the manipulator.
- Just before to touch the egg envelope surface, apply pressure to the injector by turning the handle of the injector, and then penetrate the end of the glass needle into the cytoplasm through the egg envelope and cell membrane.
Note: When the end is inserted into the cytoplasm, the injected solution automatically starts to flow into cytoplasm. If the solution doesn’t flow into the cytoplasm, apply additional pressure to the injector. - Wait a second until outflow of the solution reaches the appropriate volume, and quickly withdraw the needle (Video 3).
Note: The injection of the solution into the eggs should be stopped when the cytoplasm are expanded slightly, because over dose of the solution, especially DNA, may result in death or developmental disturbance of the eggs. The volume of the solution is not constant because the microinjection system cannot accurately control the injected volume.Video 3. The orientation of the cytoplasm toward the injection needle on the egg holder, and the microinjection of the solution into the egg (Steps 4g-4m) - Stop giving pressure after the end of the glass needle is removed completely from the egg.
- Transfer the injected eggs from the egg holder to a plastic dish filled with embryo culture medium (see Recipes).
Notes: To establish a knockout strain harboring the targeted mutagenesis, it is sufficient to inject the solution into at least 50 eggs. On the other hand, to establish a knock-in strain harboring the inserted gene, it is necessary to inject the solution into at least 100 eggs because of low efficiency in knock-in performance. It is recommended to inject the one- or two-cell stage eggs to prevent from reducing the efficiency of the injection (Video 4). - Incubate the injected eggs in embryo culture medium at around 28 °C.Video 4. The reference movie of the microinjection into the two-cell stage eggs. Unlike the egg holder with a silicone channel described in this protocol, the egg is fitted a channel between glass plates in this movie. The first injection is performed with the one-cell stage egg, while the second injection is performed with the two-cell stage egg. It is recommended that the solution be injected into the one-cell stage eggs, because the two-cell stage eggs are required to be injected into each cytoplasm as shown in the second injection in this video. It should be noted that the microinjection into the eggs later than two-cell stage strongly reduces the efficiency of the injection, and leads to a high degree of mosaicism in the eggs. The injected solution gives a red color with a phenol red, so that can be visible in the egg in this movie, however, it is unnecessary for the practical experiment.
- Remove the partition in the fish tank to make the pairs begin spawning.
Recipes
- 17 mM methylene blue (methylene blue stock solution)
Add 50 ml of purified water to 3.2 x 102 mg of methylene blue
Note: Can be stored at RT for several months. - 100x embryo culture medium
1.7 M NaCl
40 mM KCl
27 mM CaCl2·2H2O
65 mM MgSO4·7H2O
Note: Can be stored at RT for several months. - Embryo culture medium
After dilution of 100x embryo culture medium with purified water, add 1 drop of the methylene blue solution per 1 L of the diluted solution
Acknowledgments
This protocol was adapted from our previous works (Murakami et al., 2017a). The authors declare no conflicts of interest or competing interests with this manuscript.
References
- Ansai, S. and Kinoshita, M. (2014). Targeted mutagenesis using CRISPR/Cas system in medaka. Biol Open 3(5): 362-371.
- Kinoshita, M., Murata, K., Naruse, K. and Tanaka, M. (2009). Medaka: Biology, management, and experimental protocols. Wiley-Blackwell 280-281.
- Murakami, Y., Ansai, S., Yonemura, A. and Kinoshita, M. (2017a). An efficient system for homology-dependent targeted gene integration in medaka (Oryzias latipes). Zoological Lett 3: 10.
- Murakami, Y., Ansai, S., Yonemura, A. and Kinoshita, M. (2017b). Genotyping-free selection of double allelic gene edited medaka using two different fluorescent proteins. Bio-protocol e2665.
- Ozato, K., Kondoh, H., Inohara, H., Iwamatsu, T., Wakamatsu, Y. and Okada, T. S. (1986). Production of transgenic fish: introduction and expression of chicken delta-crystallin gene in medaka embryos. Cell Differ 19(4): 237-244.
Article Information
Copyright
© 2018 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Murakami, Y. and Kinoshita, M. (2018). Medaka-microinjection with an Upright Microscope. Bio-protocol 8(3): e2716. DOI: 10.21769/BioProtoc.2716.
Category
Molecular Biology > DNA > Mutagenesis
Cell Biology > Cell engineering > Microinjection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










